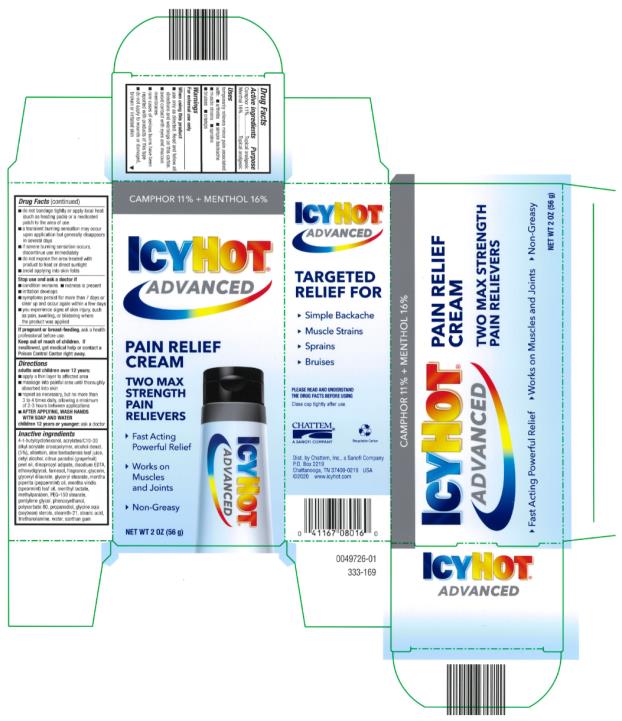
Uses
Temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
Warnings
For external use only
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad, or medicated patch
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- a transient burning sensation may occur upon application but generally disappears within a few days
Directions
adults and children over 12 years:
- apply a thin layer to affected area
- message into painful area until thoroughly absorbed into skin
- repeat as necessary, but no more than 3 to 4 times daily, allowing a minimum of 2-3 hours between applications
- AFTER APPLYING, WASH HANDS WITH SOAP AND WATER
Children 12 years and younger: ask a doctor
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, allantoin, aloe barbadensis leaf juice, cetyl alcohol, diisopropyl adipate, disodium EDTA, ethoxydiglycol, fragrance, glycerin, glyceryl stearate, menthyl lactate, methylparaben, PEG-150 sterate, pentylene glycol, 4-t-butylcyclohexanol, phenoxyethanol, polysorbate 80, propanediol, SD alcohol (5% w/w), glycine soja (soybean) sterols, steareth-21, stearic acid, triethanolamine, water, xanthan gum (309-018)