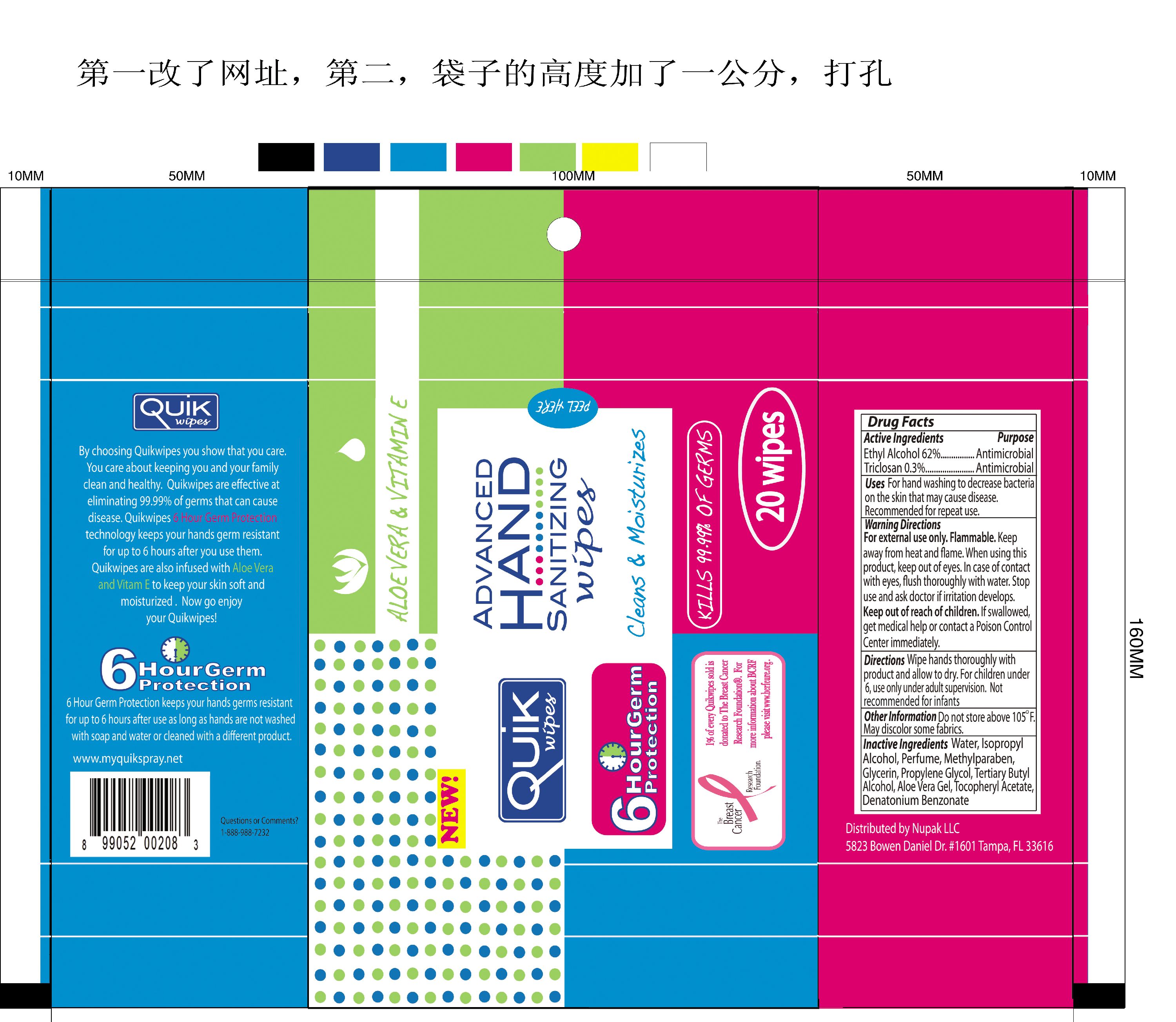
Active Ingredients Purpose
Ethyl Alcohol 62 percent Antimicrobial
Triclosan 0.3 percent Antimicrobial
Uses For hand washing to decrease bacteria on the skin that may cause disease.
Recommended for repeat use.
Warning Directions
For external use only. Flammable.
Keep away from heat and flame. When using this product, keep out of eyes. In case of contact with eyes, flush thoroughly with water.
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center immediately.
Directions Wipe hands thoroughly with product and allow to dry.
For children under 6, use only under adult supervision.
Not recommended for infants