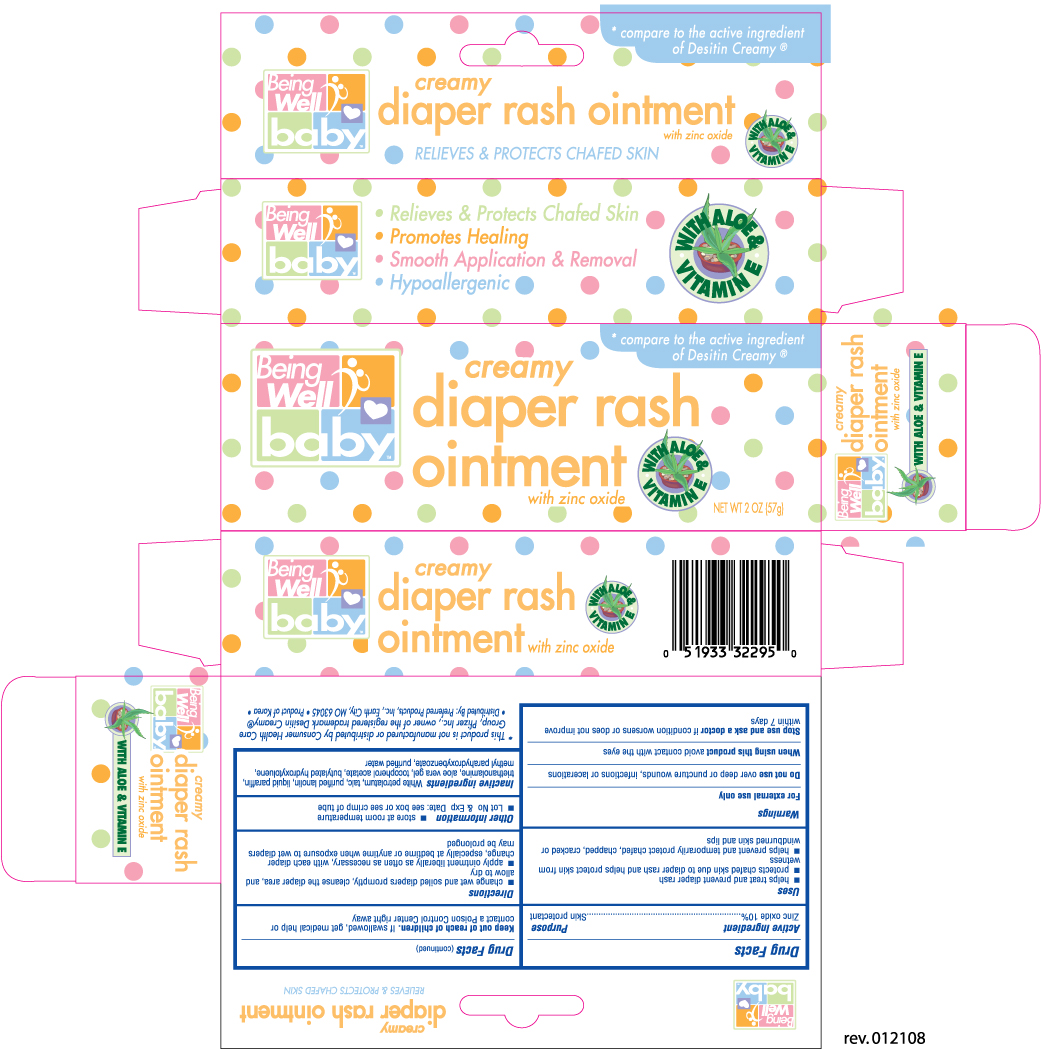
BEING WELL DIAPER RASH CREAMY - zinc oxide ointment
TAI GUK PHARM. CO., LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient Purpose
Zinc oxide 10% ........................................................................ Skin protectant
Uses
- helps treat and prevent diaper rash
- protects chafed skin due to diaper rash and helps protect skin from wetness
- helps prevent and temporarily protect chafed, chapped, cracked or windburned skin and lips
Warnings
For external use only
Do not use over deep or puncture wounds, infections or lacerations
When using this product avoid contact with the eyes
Stop use and ask a doctor if condition worsens or does not improve within 7 days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
- change wet and soiled diapers promptly, cleanse the diaper area and allow to dry
- apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
Other information
- store at room temperature
- Lot No and Exp. Date: see box or see crimp of tube
Inactive ingredients
White petrolatum, talc, purified lanolin, liquid paraffin, triethanolamine, aloe vera gel, tocopherol acetate, butylated hydroxyloluene, methyl parahydroxybenzoate, purified water
Distributed by: Preferred Products, Inc.
Earth City, MO 63045
Product of Korea
 Enter section text here
Enter section text here
TAI GUK PHARM. CO., LTD.
 Enter section text here
Enter section text here