DESCRIPTION
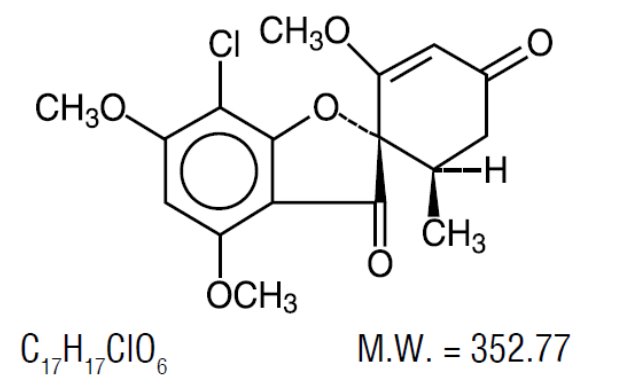
Griseofulvin tablets, USP (microsize) contains griseofulvin microsize for oral administration. The active ingredient, griseofulvin, is a fungistatic antibiotic, derived from a species of Penicillium. The chemical name of griseofulvin is 7-chloro-2’,4,6-trimethoxy-6’β-methylspiro[benzofuran-2(3H),1’-[2]cyclohexane]-3-4’-dione. Its structural formula is:
Griseofulvin occurs as a white to creamy white, bitter tasting powder which is very slightly soluble in water and sparingly soluble in alcohol. Griseofulvin microsize contains particles of approximately 2 to 4 µm in diameter.
Griseofulvin tablets, USP (microsize) are available as follows:
250 mg, white to off-white round, flat, beveled edge, scored tablets engraved with 'I27' on one side and score on the other.
500 mg, white to off-white round, flat, beveled edge, scored tablets engraved with 'I26' on one side and score on the other.
Each tablet contains 250 mg or 500 mg of griseofulvin microsize, and also contains calcium stearate, colloidal silicon dioxide, corn starch, crospovidone, dibasic calcium phosphate, and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Griseofulvin absorption from the gastrointestinal tract varies considerably among individuals, mainly because of insolubility of the drug in aqueous media of the upper GI tract. Drug absorption has been estimated to range between 27 and 72%. After an oral dose, griseofulvin is primarily absorbed from the duodenum with some absorption occurring from the jejunum and ileum. The peak serum level in fasting adults given 0.5 g of griseofulvin microsize occurs at about four hours and ranges between 0.5 to 2 mcg/mL. The serum level may be increased by giving the drug with a meal with a high fat content. In one study in pediatric patients 19 months to 11 years of age, 10 mg/kg of griseofulvin microsize given with milk resulted in mean peak serum concentrations approximately four-fold greater than the same griseofulvin dose given alone (1.29 mcg/mL versus 0.34 mcg/mL, respectively). Also, the area under the curve value was ten-fold larger when 10 mg/kg griseofulvin and milk were administered simultaneously as compared to the same dosage given to fasting patients. In addition, griseofulvin administered with milk resulted in more consistently detected serum levels across subjects.
Following oral administration, griseofulvin is deposited in the keratin precursor cells and has a greater affinity for diseased tissue. The drug is tightly bound to the new keratin which becomes highly resistant to fungal invasions. When the drug is discontinued, griseofulvin concentrations in the skin decline less rapidly than those in plasma. Griseofulvin is metabolized by the liver to 6-desmethylgriseofulvin and its glucuronide conjugate.
Griseofulvin has a variable elimination half-life in plasma (9 to 24 hours). Approximately 30% of a single oral dose of griseofulvin is excreted in the urine within 24 hours and about 50% of the dose is excreted in the urine within 5 days, mostly in the form of metabolites. Unchanged griseofulvin in the urine accounts for less than 1% of the administered dose. In addition, approximately one-third of a single dose of griseofulvin is excreted in feces within 5 days. Griseofulvin is also excreted in perspiration.
Microbiology
Mechanism of Action
The mechanism of griseofulvin consists of binding microtubular proteins, which are required for mitosis.
Activity In Vivo
Griseofulvin may be active against most strains of the following dermatophytes as described in the INDICATIONS AND USAGE section:
Epidermophyton floccosum, Microsporum audouinii, Microsporum canis, Microsporum gypseum, Trichophyton crateriformis, Trichophyton gallinae, Trichophyton interdigitalis, Trichophyton megnini, Trichophyton mentagrophytes, Trichophyton rubrum, Trichophyton sulphureum, Trichophyton schoenleini, Trichophyton tonsurans and Trichophyton verrucosum.
It has no effect on bacteria or on other genera of fungi.
INDICATIONS AND USAGE
Griseofulvin tablets, USP are indicated for the treatment of dermatophyte infections of the skin not adequately treated by topical therapy, hair and nails, namely:
Tinea corporis
Tinea pedis
Tinea cruris
Tinea barbae
Tinea capitis
Tinea unguium when caused by one or more of the following species of fungi:
Epidermophyton floccosum
Microsporum audouinii
Microsporum canis
Microsporum gypseum
Trichophyton crateriform
Trichophyton gallinae
Trichophyton interdigitalis
Trichophyton megnini
Trichophyton mentagrophytes
Trichophyton rubrum
Trichophyton schoenleini
Trichophyton sulphureum
Trichophyton tonsurans
Trichophyton verrucosum
Note: Prior to therapy, a dermatophyte should be identified as responsible for the infection.
Prior to initiating treatment, appropriate specimens for laboratory testing (KOH preparation, fungal culture, or nail biopsy) should be obtained to confirm the diagnosis.
Griseofulvin tablets, USP are not effective in the following:
Bacterial infections
Candidiasis (Moniliasis)
Histoplasmosis
Actinomycosis
Sporotrichosis
Chromoblastomycosis
Coccidioidomycosis
North American Blastomycosis
Cryptococcosis (Torulosis)
Tinea versicolor
Nocardiosis
The use of this drug is not justified in minor or trivial dermatophyte infections which will respond to topical agents alone.
CONTRAINDICATIONS
Griseofulvin is contraindicated in patients with porphyria or hepatocellular failure, and in individuals with a history of hypersensitivity to griseofulvin.
Griseofulvin may cause fetal harm when administered to a pregnant woman. Two published cases of conjoined twins have been reported in patients taking griseofulvin during the first trimester of pregnancy, therefore, griseofulvin is contraindicated in women who are or may become pregnant during treatment. Women taking estrogen-containing oral contraceptives may be at increased risk of becoming pregnant while on griseofulvin (see also PRECAUTIONS, Drug Interactions). If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Although no direct causal relationship has been established, spontaneous abortion has been reported rarely coincident with the use of griseofulvin.
Note: The Maximum Recommend Human Dose (MRHD) was set at 500 mg/day for the multiple of human exposure calculations performed in this label. If higher doses than 500 mg/day were used clinically, then the multiple of human exposure would be correspondingly reduced for that dose. For example, if a 1000 mg/day dose was administered to an individual, then the multiple of human exposure would be reduced by a factor of 2.
Griseofulvin has been shown to be embryotoxic and teratogenic in pregnant rats when given at a daily oral dose of 250 mg/kg/day [4X the Maximum Recommended Human Dose (MRHD) based on Body Surface Area (BSA)]. Griseofulvin also has been shown to be embryotoxic and teratogenic in pregnant cats treated weekly with griseofulvin at doses of 500 to 1000 mg/week. There are reports of teratogenicity in a Golden Retriever when doses of 750 mg/day [1.2X the MRHD based on BSA] were administered for four weeks prior to and throughout the pregnancy, and in a study in which beagles were administered 35 mg/kg/day [1.9X the MRHD based on BSA] for intervals from one week up to the entire gestation period. Teratogenicity was also seen in mice when griseofulvin was administered in doses equivalent to 5g/kg/day [40X the MRHD based on BSA] for 2 consecutive days at various stages of the pregnancy.
WARNINGS
Prophylactic Usage
Safety and efficacy of griseofulvin for prophylaxis of fungal infections have not been established.
Serious Skin Reactions
Severe skin reactions (e.g. Stevens-Johnson syndrome, toxic epidermal necrolysis) and erythema multiforme have been reported with griseofulvin use. These reactions may be serious and may result in hospitalization or death. If severe skin reactions occur, griseofulvin should be discontinued (see ADVERSE REACTIONS section).
Hepatotoxicity
Elevations in AST, ALT, bilirubin, and jaundice have been reported with griseofulvin use. These reactions may be serious and may result in hospitalization or death. Patients should be monitored for hepatic adverse events and discontinuation of griseofulvin considered if warranted (see ADVERSE REACTIONS section).
PRECAUTIONS
General
Patients on prolonged therapy with any potent medication should be under close observation. Periodic monitoring of organ system function, including renal, hepatic and hematopoietic, should be done.
Since griseofulvin is derived from species of penicillin, the possibility of cross sensitivity with penicillin exists; however, known penicillin-sensitive patients have been treated without difficulty.
Lupus erythematosus, lupus-like syndromes or exacerbation of existing lupus erythematosus have been reported in patients receiving griseofulvin.
Since a photosensitivity reaction is occasionally associated with griseofulvin therapy, patients should be warned to avoid exposure to intense or prolonged natural or artificial sunlight.
Drug Interactions
Griseofulvin has been reported in the literature to interfere with the metabolism of various compounds. Whether this is due to a P-450 mediated enzyme induction effects on sulfurtransferase and/or glucotransferase activity, or some other mechanism is unknown.
Griseofulvin decreases the activity of warfarin-type anticoagulants, so that patients receiving these drugs concomitantly may require dosage adjustment of the anticoagulant during and after griseofulvin therapy.
Griseofulvin may enhance the hepatic metabolism of estrogens, including the estrogen component of oral contraceptives, thereby reducing the effectiveness of contraception and causing menstrual irregularities. Therefore, an alternate or second form of birth control may be indicated during periods of concurrent use (see also CONTRAINDICATIONS).
Cyclosporine levels may be reduced when administered concomitantly with griseofulvin, resulting in a decrease in the pharmacologic effects of cyclosporine.
Serum salicylate concentrations may be decreased when griseofulvin is given concomitantly with salicylates.
Barbiturates usually depress griseofulvin activity by decreasing plasma levels and concomitant administration may require a dosage adjustment of the antifungal agent.
Nausea, vomiting, flushing, tachycardia, and severe hypotension have been reported following alcohol ingestion during griseofulvin therapy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In subacute toxicity studies, orally administered griseofulvin produced hepatocellular necrosis in mice, but this has not been seen in other species. Chronic feeding of griseofulvin, at levels ranging from 0.5 to 2.5% of the diet, resulted in the development of liver tumors in several strains of mice, particularly in males. Smaller particle sizes resulted in an enhanced effect. Lower oral-dosage levels have not been tested.
Subcutaneous administration of relatively small doses of griseofulvin once a week during the first three weeks of life has also been reported to induce hepatomata in mice. Thyroid tumors, mostly adenomas but some carcinomas, have been reported in male rats receiving griseofulvin at levels of 2%, 1%, and 0.2% of the diet, and in female rats receiving the two higher dose levels. Studies in other animal species were inadequate assessments of tumorigenicity.
Disturbances in porphyrin metabolism have been reported in griseofulvin-treated laboratory animals. Griseofulvin has been reported to have a colchicine-like effect on mitosis and was cocarcinogenic with methylcholanthrene in cutaneous tumor induction in laboratory animals. Griseofulvin interferes with chromosomal distribution during cell division, causing aneuploidy in plant and mammalian cells. These effects have been demonstrated in vitro at concentrations that may be achieved in the serum with the recommended therapeutic dosage.
Suppression of spermatogenesis has been reported to occur in rats and sperm abnormalities have been observed in griseofulvin treated mice, but these were not detected in man. Male patients should wait at least six months after completing griseofulvin therapy before fathering a child.
Nursing Mothers
It is not known if griseofulvin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for tumorigenicity shown for griseofulvin in animal studies (see PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility), a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
There have been post-marketing reports of severe skin and hepatic adverse events associated with griseofulvin use (see WARNINGS section).
When adverse reactions occur, they are most commonly of the hypersensitivity type, such as skin rashes, urticaria, and rarely, angioneurotic edema, and erythema multiforme. These may necessitate withdrawal of therapy and appropriate countermeasures.
Peripheral neuropathy and paresthesias of the hands and feet have been reported and may be related to treatment duration. Most patients treated with griseofulvin for less than six months experienced improvement or resolution of their neuropathy upon withdrawal of the griseofulvin. Other side effects reported occasionally are oral thrush, nausea, vomiting, epigastric distress, diarrhea, headache, fatigue, dizziness, insomnia, mental confusion and impairment of performance of routine activities.
Proteinuria, nephrosis (sometimes associated with existing systemic lupus erythematosus), leukopenia, coagulopathy, hepatitis, elevated liver enzymes, hyperbilirubinemia, and GI bleeding have been reported rarely. Administration of the drug should be discontinued if granulocytopenia occurs.
OVERDOSAGE
There is limited experience on overdose with griseofulvin. In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures as required.
DOSAGE AND ADMINISTRATION
Accurate diagnosis of the infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide or by culture on an appropriate medium.
Medication must be continued until the infecting organism is completely eradicated as indicated by appropriate clinical or laboratory examination. Representative treatment periods are tinea capitis, 4 to 6 weeks; tinea corporis, 2 to 4 weeks; tinea pedis, 4 to 8 weeks; tinea unguium – depending on rate of growth – fingernails, at least 4 months; toenails, at least 6 months.
General measures in regard to hygiene should be observed to control sources of infection or reinfection. Concomitant use of appropriate topical agents is usually required, particularly in treatment of tinea pedis. In some forms of tinea pedis, yeasts and bacteria may be involved as well as dermatophytes. Griseofulvin tablets, USP will not eradicate these associated bacterial or yeast infections.
ADULTS: 0.5 g daily (125 mg q.i.d., 250 mg b.i.d., or 500 mg/day). Patients with less severe or 300 extensive infections may require less, whereas those with widespread lesions may require a starting dose of 0.75 g to 1 g/day. This may be reduced gradually to 0.5 g or less after a response has been noted. In all cases, the dosage should be individualized.
PEDIATRIC PATIENTS (older than 2 years): A dosage of 10 mg/kg daily is usually adequate (pediatric patients from 30 to 50 lb, 125 mg to 250 mg daily; pediatric patients over 50 lb, 250 mg to 500 mg daily, in divided doses). Dosage should be individualized, as with adults. Clinical relapse will occur if the medication is not continued until the infecting organism is eradicated.
Safety is not established at higher doses than recommended.
HOW SUPPLIED
Griseofulvin tablets, USP (microsize) are available as follows:
250 mg, white to off-white round, flat, beveled edge, scored tablets engraved with 'I27' on one side and score on the other.
- NDC 0781-5514-01, in bottles of 100 tablets
- NDC 0781-5514-05, in bottles of 500 tablets
500 mg, white to off-white round, flat, beveled edge, scored tablets engraved with 'I26' on one side and score on the other.
- NDC 0781-5515-01, in bottles of 100 tablets
- NDC 0781-5515-05, in bottles of 500 tablets
Dispense griseofulvin tablets, USP (microsize) in a tight container as defined in the USP.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Manufactured by
USV Private Limited,
Mahatma Gandhi Udyog Nagar,
Dabhel, Daman 396210, India
for Sandoz Inc.,
Princeton, NJ 08540
Rev. December 2016