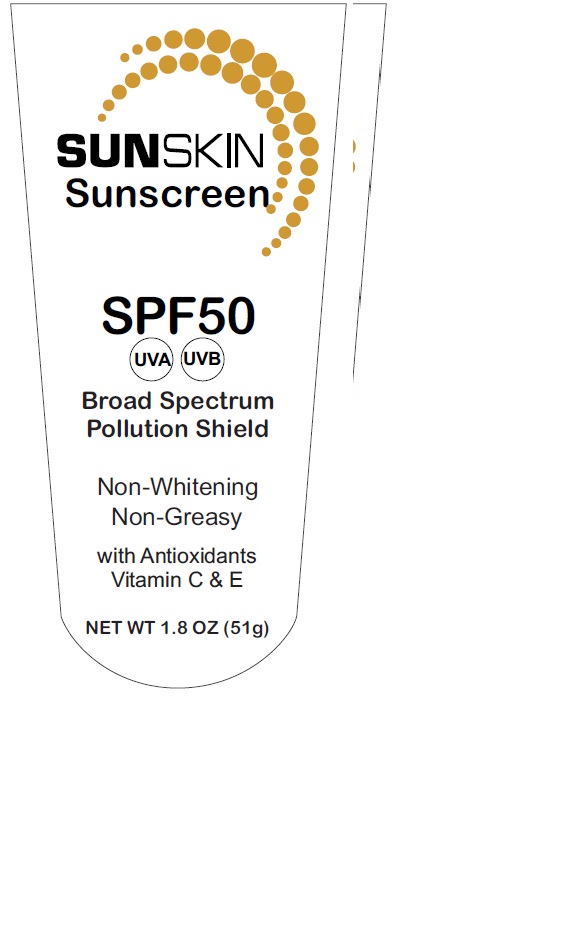
PURPOSE
Sunscreen
Uses
● helps prevent sunburn
● if used as directed with other sun protection measures
- decreases the risk of skin cancer and early skin aging caused by the sun
Active ingredients
Avobenzone 3%, Homosalate 11.7%,
Octisalate 4.5%, Oxybenzone 5.4%,
Octocrylene 4.5%
Directions
● apply generously 15 minutes before sun exposure
● ensure complete coverage to the are above the lip, nose, and tops of ears
● reapply: - after 80 minutes of swimming or sweating – immediately after towel drying – at least every 2 hours.
WARNING AND Pracaution
●For external use only
●Do not use on damaged or broken skin
●When using this product keep out of eyes. Rinse with water to remove.
●If rash occurs, stop use and ask a doctor
KEEP OUT OF REACH OF CHILDREN SECTION
- KEEP OUT OF REACH OF CHILDREN SECTION
- If swallowed, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENT
ingredients: water, propylene glycol,
polyurethane-34, cetyl alcohol, potassium cetyl
phosphate, glycerin, sorbitan mono stearate,
polysorbate 60, phenoxyethanol,
ethylhexylglycerin, potassium hydroxide,
dimethicone, tocopheryl acetate, acrylates/C10-
30 alkyl acrylate crosspolymer, arginine, sodium
ascorbyl phosphate, disodium EDTA