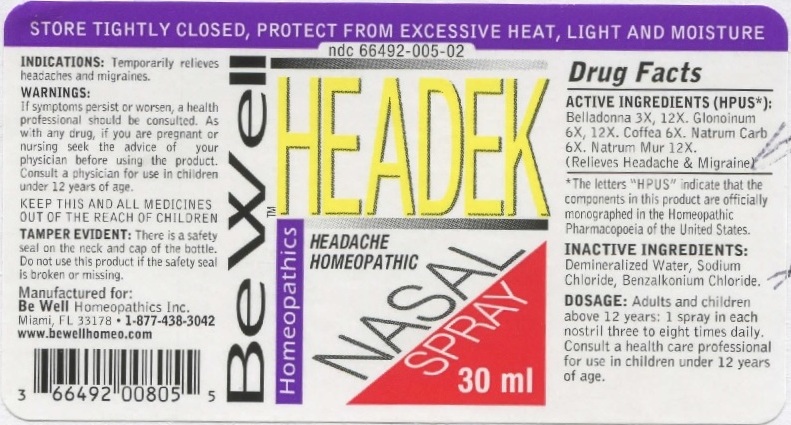
HEADEK NASAL- belladonna, glonoinum, coffea, natrum carb, natrum mur. spray
Be Well Medical dba Richard Clement Nutrition y Be Well Homeopathics
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS (HPUS*): Belladonna 3X, 12X. Glonoinum 6X, 12X. Coffea 6X. Natrum Carb 6X. Natrum Mur 12X. (Relieves Headache & Migraine)
*The letters "HPUS" indicate that the components in the product are officially monographed in the Homeopathic Pharmacopeia of the United States.
INDICATIONS: Temporarily relieves headaches and migraines.
WARNINGS: If symptoms persist or worsen, a health professional should be consulted. As with any drug, if you are pregnant or nursing seek the advice of your physician before using this product. Consult a physician for use in children under 12 years of age.
Keep this and all medicines out of the reach of children.
DOSAGE: Adults and children above 12 years: 1 spray in each nostril three to eight times daily. Consult a health care professional for use in children under 12 years of age.
TAMPER EVIDENT: There is a safety seal on the neck and cap of the bottle. Do not use this product if the safety seal is broken or missing.
INACTIVE INGREDIENTS: Demineralized Water, Sodium Chloride, Benzalkonium Chloride.
Manufactured for: Be Well Homeopathics Inc. Miami, FL 33178 • 1-877-438-3042
www.bewellhomeo.com
NDC 66492-005-02
Be Well Homeopathics
HEADEK NASAL SPRAY
30ml