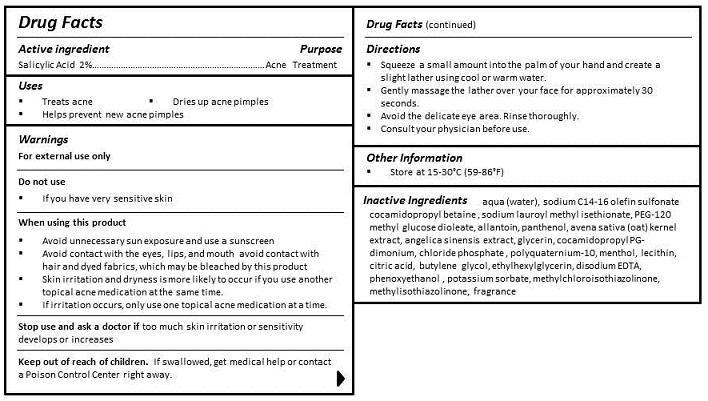
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- Squeeze a small amount into the palm of your hand and create a slight lather using cool or warm water.
- Gently massage the lather over your face for approximately 30 seconds.
- Avoid the delicate eye area. Rinse thoroughly.
- Consult your physician before use.
INGREDIENTS
aqua (water), sodium C14-16 olefin sulfonate cocamidopropyl betaine , sodium lauroyl methyl isethionate, PEG-120 distearate, allantoin, panthenol, avena sativa (oat) kernel extract, angelica sinensis extract, glycerin, cocamidopropyl PG-dimonium, chloride phosphate, polyquaternium-10, menthol, lecithin, citric acid, butylene glycol, ethylhexylglycerin, disodium EDTA, phenoxyethanol, potassium sorbate, methylchloroisothiazolinone, methylisothiazolinone, fragrance