DESCRIPTION
Virt-Caps is an orally administered prescription vitamin B dietary supplement formulated for the dietary management of persons who require increased amounts of folic acid and pyridoxine; it also includes vitamin C. Virt-Caps should be administered under the supervision of a licensed medical practitioner.
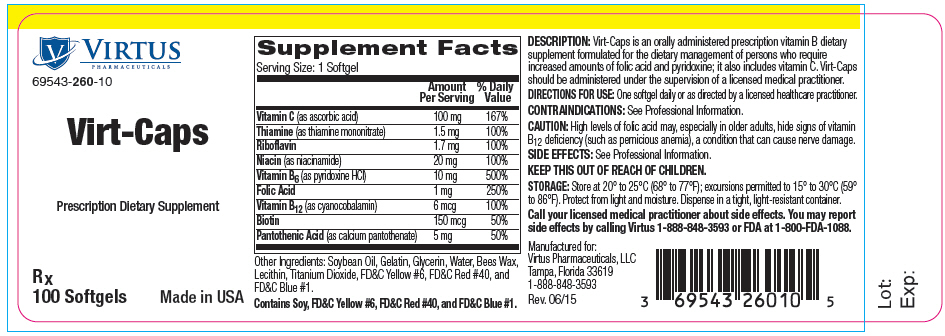
| Supplement Facts Serving Size: 1 Softgel |
||
|---|---|---|
| Amount Per Serving | % Daily Value | |
| Vitamin C (as ascorbic acid) | 100 mg | 167% |
| Thiamine (as thiamine mononitrate) | 1.5 mg | 100% |
| Riboflavin | 1.7 mg | 100% |
| Niacin (as niacinamide) | 20 mg | 100% |
| Vitamin B6 (as pyridoxine HCl) | 10 mg | 500% |
| Folic Acid | 1 mg | 250% |
| Vitamin B12 (as cyanocobalamin) | 6 mcg | 100% |
| Biotin | 150 mcg | 50% |
| Pantothenic Acid (as calcium pantothenate) | 5 mg | 50% |
Other Ingredients: Soybean Oil, Gelatin, Glycerin, Water, Bees Wax, Lecithin, Titanium Dioxide, FD&C Yellow #6, FD&C Red #40, and FD&C Blue #1.
Contains Soy, FD&C Yellow #6, FD&C Red #40 and FD&C Blue #1.
Side Effects
Allergic reactions have been reported following the use of oral folate.
Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body has been associated with cobalamin.
Paresthesia, somnolence, nausea, and headaches have been reported with pyridoxine.
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN.
INTERACTIONS
Drugs which may interact with folate include but are not limited to:
- First generation anticonvulsants (folate may reduce their effectiveness)
- Capecitabine (folate may enhance its toxicity)
- Fluorouracil (folate may enhance its toxicity)
Drugs that may interfere with folate metabolism include, but are not limited to:
- Dihydrofolate Reductase Inhibitors
- Fluoxetine
- Nonsteroidal Anti-inflammatory Drugs
- Sulfasalazine
- Warfarin
Drugs which may interfere with the absorption of folate from the gastrointestinal tract or decrease plasma levels include, but are not limited to:
- Cholestyramine
- Colestipol
- Colchicine
- Levodopa
- Cycloserine
- Isotretinoin
- Oral Contraceptives
- Methylprednisolone
- Pancreatic Enzymes
- Pentamidine
- Smoking and Alcohol
- Sulfasalazine
- Metformin
Drugs which may interact with vitamin B6 include, but are not limited to:
- Levodopa (the action of levodopa may be antagonized by vitamin B6.)
Drugs which may interact with vitamin B12 include, but are not limited to:
- Antibiotics, cholestyramine, colchicine, colestipol, metformin, para-aminosalicylic acid, and potassium chloride (may decrease the absorption of vitamin B12)
- Nitrous oxide (can produce a functional vitamin B12 deficiency)
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of its components.
CAUTION
High levels of folic acid may, especially in older adults, hide signs of vitamin B12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
PREGNANCY and NURSING MOTHERS
This product contains only B and C vitamins and is not intended for use as a prenatal/postnatal multivitamin.
STORAGE
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). Protect from light and moisture. Dispense in a tight, light-resistant container. Notice: Contact with moisture may produce surface discoloration or erosion.
HOW SUPPLIED
Virt-Caps is supplied as black softgels imprinted on one side with "V260" dispensed in child-resistant bottles of 100 softgels
69543-260-10
KEEP THIS OUT OF REACH OF CHILDREN.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Call your medical practitioner about side effects. You may report side effects by calling Virtus at 1-888-848-3593 or FDA at 1-800-FDA-1088.
Rx
Manufactured for:
Virtus Pharmaceuticals, LLC
Tampa, Florida 33619
1-888-848-3593
MADE IN USA
Rev. 06/2015