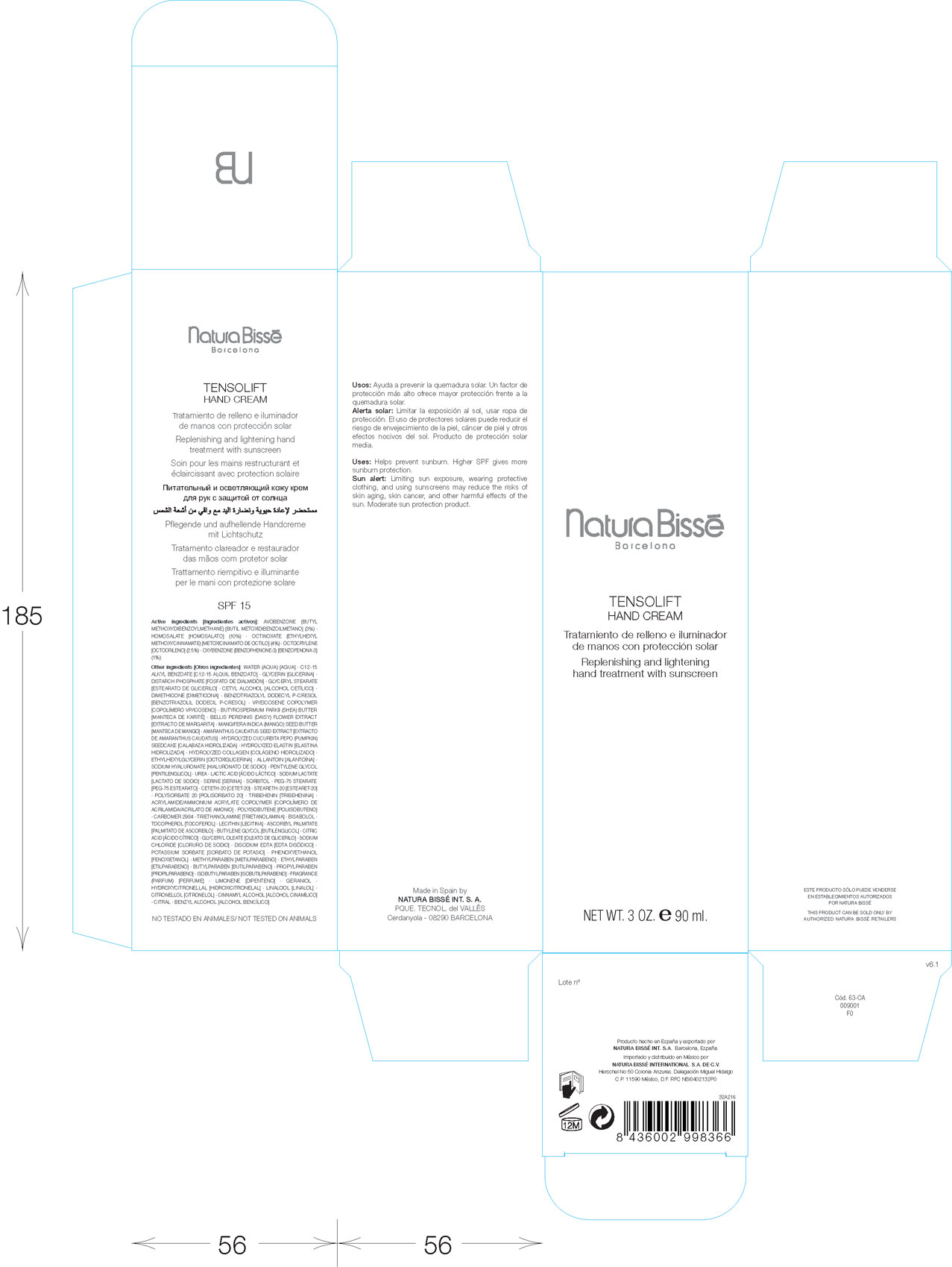
Active Ingredients: Avobenzone (Butyl Methoxydibenzoylmethane) 3 percent. Homosalate 10 percent. Octinoxate (Ethylhexyl Methoxycinnamate) 4 percent. Octocrylene 2.5 percent. Oxybenzone (Benzophenone-3) 1 percent.
Other Ingredients: Water (Aqua). C12-15 Alkyl Benzoate. Glycerin. Distarch Phsphate. Glyceryl Stearate. Cetyl Alcohol. Dimethicone. Benzotriazolyl Dodecyl P-Cresol. VP/Eicosene Copolymer. Butyrospermum Parkii (SHEA) Butter. Amaranthus Caudatus Seed Extract. Hydrolyzed Cucurbita Pepo (Pumkin) Seedcake. Hydrolyzed Elastin. Hydrolyzed Collagen. Ethylhexylglycerin. Allantoin. Sodium Hyaluronate. Pentylene Glycol. Urea. Lactic Acid. Sodium Lactate. Serine. Sorbitol. PEG-75 Stearate. Ceteth-20. Steareth-20. Polysorbate 20. Tribehenin. Acrylamide/Ammoniu, Acrylate Copolymer. Polyisobutene. Carbomer 2984. Trietholamine. Bisabolol. Tocopherol. Lecithin. Ascorbyl Palmitate. Butylene Glycol. Citric Acid. Glyceryl Oleate. Sodium Chloride. Disodium Edta. Potassium Sorbate. Phynoxyethanol. Methylparaben. Ethylparaben. butylparaben. Propylparaben. Isobutylparaben. Fragrance (Parfum). Limonene. Geraniol. Hydroxycitronellal. Linalool. Citronellel. Cinnamyl Alcohol. Citral. Benzyl Alcohol.
Uses: Helps prevent sunburn. Higher SPF gives more sunburn protection.
Sun Alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun. Moderate sun protection product.
Sun Alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun. Moderate sun protection product.
Natura Bisse
Barcelona
Tensolift Hand Cream
Replenishing and lightening hand treatment with sunscreen
Net Wt. 3 Oz. 90 ml.
Made in Spain by
Natura Bisse Int. S.A.
Cerdanyola - 08290 Barcelona
Herschel No 50 Colonia Anzures. Delegaciun Miguel Hidalgo.
C.P. 11590 Mexico, D.F. RFC NBI0402132P0
Not tested on animals
Barcelona
Tensolift Hand Cream
Replenishing and lightening hand treatment with sunscreen
Net Wt. 3 Oz. 90 ml.
Made in Spain by
Natura Bisse Int. S.A.
Cerdanyola - 08290 Barcelona
Herschel No 50 Colonia Anzures. Delegaciun Miguel Hidalgo.
C.P. 11590 Mexico, D.F. RFC NBI0402132P0
Not tested on animals
