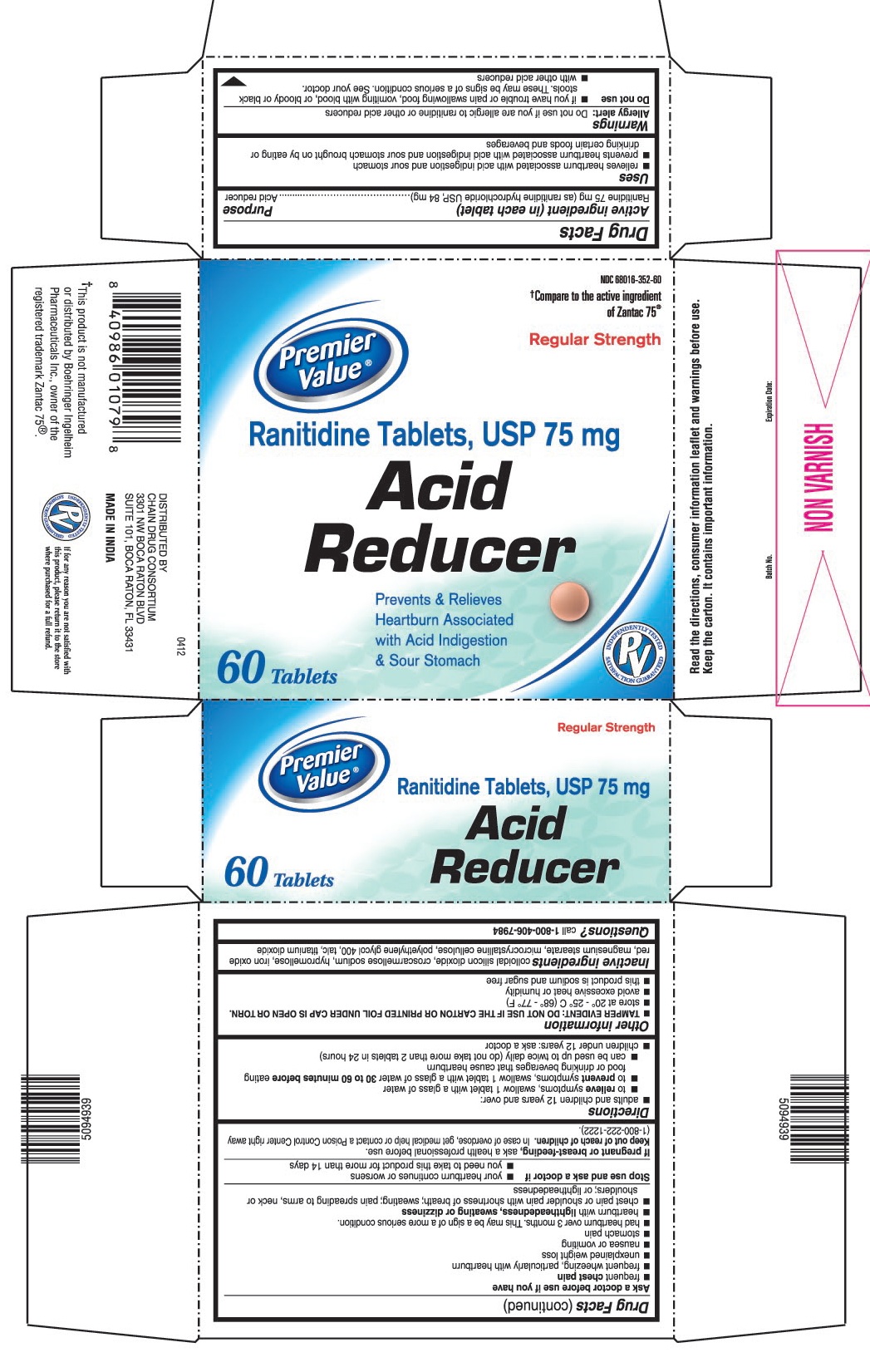
USES
- •
- relieves heartburn associated with acid indigestion and sour stomach
- •
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain foods and beverages
WARNINGS
Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- •
- with other acid reducers
Ask a doctor before use if you have
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
DIRECTIONS
- •
- adults and children 12 years and over:
- •
- to relieve symptoms, swallow 1 tablet with a glass of water
- •
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- •
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- •
- children under 12 years: ask a doctor
OTHER INFORMATION
- •
- TAMPER EVIDENT: DO NOT USE IF THE CARTON OR PRINTED FOIL UNDER CAP IS OPEN OR TORN.
- •
- store at 20° - 25° C (68° - 77° F)
- •
- avoid excessive heat or humidity
- •
- this product is sodium and sugar free
INACTIVE INGREDIENTS
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide red, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, talc, titanium dioxide
QUESTIONS?
call 1-800-406-7984
Read the directions, consumer information leaflet and warnings before use.
Keep the carton. It contains important information.
DISTRIBUTED BY
CHAIN DRUG CONSORTIUM
3301 NW BOCA RATON BLVD
SUITE 101, BOCA RATON, FL 33431
PRINCIPAL DISPLAY PANEL
Premier Value®
NDC 68016-352-60
Ranitidine Tablets, USP 75 mg
Acid Reducer
Regular Strength
60 Tablets
Prevents & Relieves Heartburn Associated with Acid Indigestion & Sour Stomach
†Compare to the active ingredient of Zantac 75®
†This product is not manufactured or distributed by Boehringer Ingelheim Pharmaceuticals Inc., owner of the registered trademark Zantac 75®.