QUEST- moxidectin gel
FDAH, Division of Wyeth
----------
QUEST®GEL
(moxidectin)
2% Equine Oral Gel
Contains 20 mg moxidectin/mL
(2.0% w/v)
DEWORMER & BOTICIDE
FOR ORAL USE IN HORSES AND PONIES
SIX MONTHS OF AGE AND OLDER
INDICATIONS
QUEST (moxidectin) 2% Equine Oral Gel when administered at the recommended dose level of 0.4 mg moxidectin/kg (2.2 lb) body weight is effective in the treatment and control of the following stages of gastrointestinal parasites in horses and ponies:
Large Strongyles
- Strongylus vulgaris – (adults and L4/L5 arterial stages)
- Strongylus edentatus – (adults and tissue stages)
- Triodontophorus brevicauda – (adults)
- Triodontophorus serratus – (adults)
Small Strongyles (adults)
- Cyathostomum spp., including
- Cyathostomum catinatum
- Cyathostomum pateratum
- Cylicostephanus spp., including
- Cylicostephanus calicatus
- Cylicostephanus goldi
- Cylicostephanus longibursatus
- Cylicostephanus minutus
- Cylicocyclus spp., including
- Cylicocyclus insigne
- Cylicocyclus leptostomum
- Cylicocyclus nassatus
- Cylicocyclus radiatus
- Coronocyclus spp., including
- Coronocyclus coronatus
- Coronocyclus labiatus
- Coronocyclus labratus
- Gyalocephalus capitatus
- Petrovinema poculatus
STRATEGIC PROTECTION PROGRAMS
Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism. For best control of parasites, all horses and ponies should be included in a strategic treatment program, with particular attention given to high performance animals, brood mares, stallions and foals. In foals, initial treatment is recommended at 6 months of age, after which they should be included in a recurrent treatment program. Because QUEST provides effective control of the mucosal stages of small strongyles (encysted cyathostomes), it is useful in reducing the frequency of treatment required for successful strategic equine parasite control. A veterinarian can assist in preparing the best program for your needs.
QUEST 2% Equine Oral Gel when used at the recommended dose rate suppresses strongyle egg production through 84 days following a single oral administration. This residual strongyle control reduces pasture contamination and provides a period of protection from reinfection for horses and ponies maintained on the same pasture.
MODE OF ACTION
QUEST 2% Equine Oral Gel acts by interfering with chloride channel-mediated neurotransmission in the parasite. This results in paralysis and elimination of the parasite. Moxidectin is safe for use in horses and ponies because it does not have the same injurious effect on the mammalian nervous system.
ADMINISTRATION AND DOSAGE
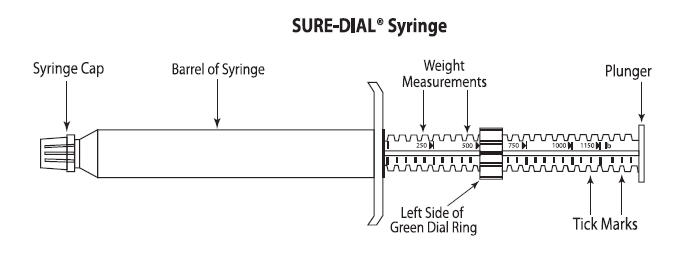
QUEST (moxidectin) 2% Equine Oral Gel is specially formulated as a palatable gel which is easily administered to horses and ponies. QUEST Gel is packaged in ready-to-use SURE-DIAL® syringes (see diagram of the SURE-DIAL® syringe below). The syringe is calibrated in 50-pound increments, up to 1150 pounds. This enables the administration of the recommended dose level of 0.4 mg moxidectin/kg body weight by choosing a setting consistent with the animal's weight.
HOW TO SET THE DOSE
Since the dose is based on the weight of the animal, you need to use a scale or weight tape to find each animal's weight before treating with QUEST Gel. Once the weight is known, set the dose for each horse or pony as follows.
- Hold the syringe with the capped end pointing to the left and so that you can see the weight measurements and tick marks (small black lines) as shown in the diagram below. Each tick mark relates to 50 lbs. of body weight.
- Turn the green dial ring until the left side of the ring lines up with the weight of the animal. In the diagram below, the dial ring is set to dose a 500 lb. animal.
HOW TO GIVE QUEST GEL TO A HORSE OR PONY
- Make sure there is no feed in the animal's mouth.
- Remove the cap from the end of the syringe. Save the cap for reuse.
- Place the tip of the syringe inside the animal's mouth at the space between the teeth.
- Gently push the plunger until it stops, depositing the gel on the back of the tongue.
- Remove the syringe from the animal's mouth and raise the animal's head slightly to make sure it swallows the gel.
- Replace the syringe cap.
TREATING A SECOND HORSE OR PONY WITH THE SAME SYRINGE
If the first animal you treat weighs less than 1150 lbs., there will be gel left in the syringe. You can use this gel to treat other horses or ponies. To set the next dose, add the weight of the animal you want to treat to the dose setting already on the syringe. For example, if the syringe was first used to treat a 250 lb. animal, the green dial ring is set on 250 lbs. To treat a 500 lb. animal next, move the green dial ring to the 750 lb. marking (250+500=750). You need more than one syringe to treat horses weighing more than 1150 lbs.
Each syringe of QUEST 2% Equine Oral Gel may be used to treat more than one animal especially when dosing ponies and growing and lighter breeds of horses. The table below will help estimate the number of horses or ponies the contents of each syringe will treat.
| Ponies | Light Horses | Heavy Horses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treated Animals | Treated Animals | Treated Animals | |||||||
| Weight | (per syringe) | Weight | (per syringe) | Weight | (per syringe) | ||||
| Age | (lbs) | (kg) | (lbs) | (kg) | (lbs) | (kg) | |||
|
|||||||||
| 6 months | 200 | (91) | 5 | 400 | (181) | 2 | 550 | (250) | 2 |
| 9 months | 250 | (113) | 4 | 500 | (227) | 2 | 700 | (318) | 1 |
| Mature | 450 | (204) | 2 | 900 | (409) | 1 | 1300 | (590) | * |
ANIMAL SAFETY
QUEST (moxidectin) 2% Equine Oral Gel can be safely administered at the recommended dose of 0.4 mg moxidectin/kg body weight to horses and ponies of all breeds at least 6 months of age or older. Transient depression, ataxia and recumbency may be seen when very young or debilitated animals are treated. In these instances, supportive care may be advisable.
Reproductive safety studies demonstrate a wide margin of safety when the product is used in the treatment of estrual and pregnant mares and breeding stallions.
To report adverse drug reactions or to obtain a copy of the Material Safety Data Sheet (MSDS) call 855-424-7349.
ENVIRONMENTAL SAFETY
Care should be taken to avoid the release of significant volumes of moxidectin into either ground or free-running water since moxidectin may be injurious to aquatic life. SURE-DIAL® syringes and their contents should be disposed of in an approved landfill or by incineration.
PRECAUTIONS
QUEST 2% Equine Oral Gel has been formulated specifically for use in horses and ponies only. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
WARNINGS
Extreme caution should be used when administering the product to foals, young and miniature horses, as overdosage may result in serious adverse reactions. Do not use in sick, debilitated, or underweight animals. Do not use in horses intended for human consumption.
HUMAN WARNINGS
Not for use in humans. Keep this and all drugs out of the reach of children. Do not ingest. If swallowed, induce vomiting. Wash hands and contaminated skin with soap and water. If accidental contact with eyes occurs, flush repeatedly with water. If irritation or any other symptom attributable to exposure to this product persists, consult your physician.
HOW SUPPLIED
QUEST 2% Equine Oral Gel is available in one syringe applicator size.
Each SURE-DIAL® syringe contains 0.4 oz (11.3 g) of QUEST 2% Equine Oral Gel which is sufficient to treat a single horse weighing up to 1150 lb, or two or more lighter animals with a combined body weight of up to 1150 lb.
NDC 0856-7441-03 — 0.4 oz (11.3 g) syringe - 20 mg moxidectin per mL
Distributed by:
Fort Dodge Animal Health
a division of Wyeth
a wholly owned subsidiary of Pfizer Inc
New York, New York 10017
Made in Spain
Revised March 2012
P1099-652US/01-12
NADA 141-087, Approved by FDA
PRINCIPAL DISPLAY PANEL - 11.3 g Syringe Label
Quest®GEL
(moxidectin)
2% Equine Oral Gel
Contains 20 mg moxidectin/mL (2.0% w/v)
0.4 oz (11.3 g) moxidectin gel
HORSE DEWORMER & BOTICIDE
FOR ORAL USE IN HORSES AND PONIES SIX MONTHS OF AGE AND OLDER
NDC 0856-7441-03
ADMINISTRATION AND DOSAGE: Each syringe treats a single horse weighing up to 1150 lb, or two or more lighter
animals with a combined body weight of up to 1150 lb. Please read entire insert for ADMINISTRATION AND DOSAGE
and other important information.
WARNINGS: Extreme caution should be used when administering the product to foals, young and miniature
horses, as overdosage may result in serious adverse reactions. Do not use in sick, debilitated, or underweight
animals. Do not use in horses intended for human consumption.
HUMAN WARNINGS: Not for use in humans. Keep this and all drugs out of the reach of children. Do not ingest.
If swallowed, induce vomiting. Wash hands and contaminated skin with soap and water. If accidental contact with
eyes occurs, flush repeatedly with water. If irritation or any other symptom attributable to exposure to this product
persists, consult your physician.
PRECAUTION: QUEST (moxidectin) Oral Gel has been formulated specifically for use in horses and ponies only. This product
should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
STORAGE: Store at or below 77°F (25°C). Avoid freezing. If frozen, thaw completely before use. Store partially-used syringe with the cap tightly secured.
NADA 141-087, Approved by FDA
Pfizer
Dist. by: Fort Dodge Animal Health
a division of Wyeth, a wholly owned
subsidiary of Pfizer Inc, NY, NY 10017
Made in Spain
Lot :
Exp. Date :
EA2116-652US/01-12

PRINCIPAL DISPLAY PANEL - 11.3 g Syringe Carton
QUEST®GEL
(moxidectin)
Controls worms and bots PLUS small strongyle larvae and eggs
For use in horses and
ponies six months of
age and older.
SURE-DIAL® syringe makes administration easy
HORSE DEWORMER
& BOTICIDE
Net Contents: One syringe
containing 0.4 oz (11.3 g)
moxidectin gel
NDC 0856-7441-03
Reduces eggs to
minimize bioburden
on pasture
Pfizer
Long-acting protection for horses

| QUEST
moxidectin gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - FDAH, Division of Wyeth (149957656) |