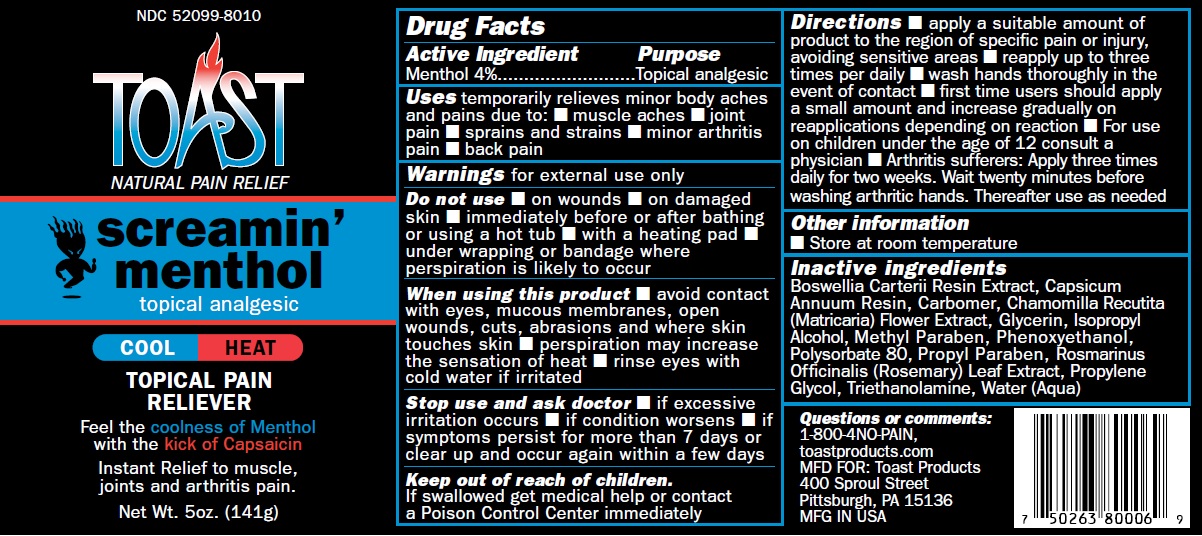
Uses temporarily relieves minor body aches and pains due to: ar thritis pain, back pain, muscle aches,joint pain.
Do not use immediately before or after bathing or using a hot tub, in conjunction with heating pad,under wrapping or bandage where perspiration is likely to occur.
Stop use and ask doctor if excessive irritation occurs, if condition worsens, if symptoms persist for more than 7 days or clear up and occur again within a few days.
When using this product avoid contact with eyes, mucous membranes, open wounds, cuts, abrasions and where skin touches skin, perspiration may increase the sensation of heat, rinse eyes with cold water if irritated.
Stop use and ask doctor if excessive irritation occurs, if condition worsens ,if symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center immediately
Directions apply a suitable amount of product to the region of specific pain or injury, avoiding sensitive areas reapply up to three times per daily, wash hands thoroughly in the event of contact. First time users should apply a small amount and increase gradually on reapplications depending on reaction. For use on children under the age of 12 consult a physician Arthritis suf ferers: Apply three times daily for two weeks. Wait twenty minutes before washing arthritic hands. Thereafter use as needed.
Inactive ingredients
Boswellia Carterii Resin Extract, Capsicum Annuum Resin, Carbomer, Chamomilla Recutita (Matricaria) Flower Extract, Glycerin, Isopropyl Alcohol, Polysorbate 80, Rosmarinus Officinalis (Rosemary) Leaf Oil, Propylene Glycol, Triethanolamine, Water (Aqua)