WARNING
Spironolactone has been shown to be a tumorigen in chronic toxicity studies in rats (see PRECAUTIONS). Spironolactone should be used only in those conditions described under INDICATIONS AND USAGE. Unnecessary use of this drug should be avoided.
DESCRIPTION
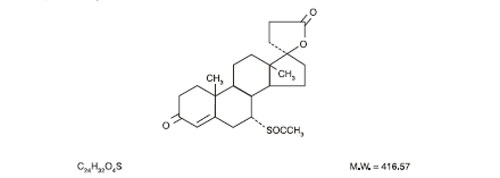
Spironolactone Tablets, USP contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate, which has the following structural formula:
Spironolactone is practically insoluble in water, soluble in alcohol, and freely soluble in benzene and in chloroform.
ACTIONS/CLINICAL PHARMACOLOGY
Mechanism of Action.
Spironolactone is a specific pharmacologic antagonist of aldosterone, acting primarily through competitive binding of receptors at the aldosterone-dependent sodium-potassium exchange site in the distal convoluted renal tubule. Spironolactone causes increased amounts of sodium and water to be excreted, while potassium is retained. Spironolactone acts both as a diuretic and as an antihypertensive drug by this mechanism. It may be given alone or with other diuretic agents that act more proximally in the renal tubule.
Aldosterone Antagonist Activity.
Increased levels of the mineralocorticoid, aldosterone, are present in primary and secondary hyperaldosteronism. Edematous states in which secondary aldosteronism is usually involved include congestive heart failure, hepatic cirrhosis, and nephrotic syndrome. By competing with aldosterone for receptor sites, spironolactone provides effective therapy for the edema and ascites in those conditions. Spironolactone counteracts secondary aldosteronism induced by the volume depletion and associated sodium loss caused by active diuretic therapy.
Spironolactone is effective in lowering the systolic and diastolic blood pressure in patients with primary hyperaldosteronism. It is also effective in most cases of essential hypertension, despite the fact that aldosterone secretion may be within normal limits in benign essential hypertension.
Through its action in antagonizing the effect of aldosterone, spironolactone inhibits the exchange of sodium for potassium in the distal renal tubule and helps to prevent potassium loss.
Spironolactone has not been demonstrated to elevate serum uric acid, to precipitate gout, or to alter carbohydrate metabolism.
Pharmacokinetics.
Spironolactone is rapidly and extensively metabolized. Sulfur-containing products are the predominant metabolites and are thought to be primarily responsible, together with spironolactone, for the therapeutic effects of the drug. The following pharmacokinetic data were obtained from 12 healthy volunteers following the administration of 100 mg of spironolactone (film-coated tablets) daily for 15 days. On the 15th day, spironolactone was given immediately after a low-fat breakfast and blood was drawn thereafter.
|
|
Accumulation
|
Mean Peak
|
Mean (SD)
|
|
7-α-(thiomethyl) |
1.25 |
391 ng/mL |
13.8 hr (6.4) |
|
6-β-hydroxy-7-α- |
1.50 |
125 ng/mL |
15.0 hr (4.0) |
|
Canrenone (C) |
1.41 |
181 ng/mL |
16.5 hr (6.3) |
|
Spironolactone |
1.30 |
80 ng/mL |
Approximately |
The pharmacological activity of spironolactone metabolites in man is not known. However, in the adrenalectomized rat the antimineralocorticoid activities of the metabolites C, TMS, and HTMS, relative to spironolactone, were 1.10, 1.28, and 0.32, respectively. Relative to spironolactone, their binding affinities to the aldosterone receptors in rat kidney slices were 0.19, 0.86, and 0.06, respectively.
In humans, the potencies of TMS and 7-α-thiospirolactone in reversing the effects of the synthetic mineralocorticoid, fludrocortisone, on urinary electrolyte composition were 0.33 and 0.26, respectively, relative to spironolactone. However, since the serum concentrations of these steroids were not determined, their incomplete absorption and/or first-pass metabolism could not be ruled out as a reason for their reduced in vivo activities.
Spironolactone and its metabolites are more than 90% bound to plasma proteins. The metabolites are excreted primarily in the urine and secondarily in bile.
The effect of food on spironolactone absorption (two 100 mg spironolactone tablets) was assessed in a single-dose study of 9 healthy, drug-free volunteers. Food increased the bioavailability of unmetabolized spironolactone by almost 100%. The clinical importance of this finding is not known.
CLINICAL STUDIES
Severe Heart Failure.
The Randomized Spironolactone Evaluation Study was a multinational, double-blind study in patients with an ejection fraction of ≤ 35%, a history of New York Heart Association (NYHA) class IV heart failure within 6 months, and class III - IV heart failure at the time of randomization. All patients were required to be taking a loop diuretic and, if tolerated, an ACE inhibitor. Patients with a baseline serum creatinine of >2.5 mg/dL or a recent increase of 25% or with a baseline serum potassium of >5.0 mEq/L were excluded.
Patients were randomized 1:1 to spironolactone 25 mg orally once daily or matching placebo. Follow-up visits and laboratory measurements (including serum potassium and creatinine) were performed every four weeks for the first 12 weeks, then every 3 months for the first year, and then every 6 months thereafter. Dosing could be withheld for serious hyperkalemia or if the serum creatinine increased to >4.0 mg/dL. Patients who were intolerant of the initial dosage regimen had their dose decreased to one tablet every other day at one to four weeks. Patients who were tolerant of one tablet daily at 8 weeks may have had their dose increased to two tablets daily at the discretion of the investigator.
The Randomized Spironolactone Evaluation Study enrolled 1,663 patients (3% U.S.) at 195 centers in 15 countries between March 24, 1995, and December 31, 1996. The study population was primarily white (87%, with 7% black, 2% Asian, and 4% other), male (73%), and elderly (median age 67). The median ejection fraction was 0.26. Seventy percent were NYHA class III and 29% class IV. The presumed etiology of heart failure was ischemic in 55%, and non-ischemic in 45%. There was a history of myocardial infarction in 28%, of hypertension in 24%, and of diabetes in 22%. The median baseline serum creatinine was 1.2 mg/dL and the median baseline creatinine clearance was 57 mL/min. The mean daily dose at study end for the patients randomized to spironolactone was 26 mg.
Concomitant medications included a loop diuretic in 100% of patients and an ACE inhibitor in 97%. Other medications used at any time during the study included digoxin (78%), anticoagulants (58%), aspirin (43%), and beta-blockers (15%).
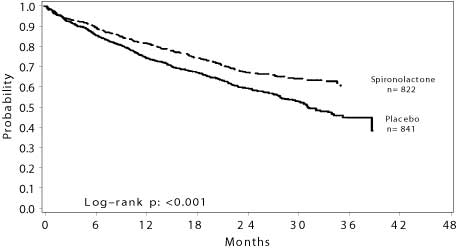
The primary endpoint for the Randomized Spironolactone Evaluation Study was time to all-cause mortality. The Randomized Spironolactone Evaluation Study was terminated early, after a mean follow-up of 24 months, because of significant mortality benefit detected on a planned interim analysis. The survival curves by treatment group are shown in Figure 1.
Figure 1. Survival by Treatment Group in The Randomized Spironolactone Evaluation Study
Spironolactone reduced the risk of death by 30% compared to placebo (p<0.001; 95% confidence interval 18% to 40%). Spironolactone reduced the risk of cardiac death, primarily sudden death and death from progressive heart failure by 31% compared to placebo (p <0.001; 95% confidence interval 18% to 42%).
Spironolactone also reduced the risk of hospitalization for cardiac causes (defined as worsening heart failure, angina, ventricular arrhythmias or myocardial infarction) by 30% (p <0.001 95% confidence interval 18% to 41%). Changes in NYHA class were more favorable with spironolactone: In the spironolactone group, NYHA class at the end of the study improved in 41% of patients and worsened in 38% compared to improved in 33% and worsened in 48% in the placebo group (p <0.001).
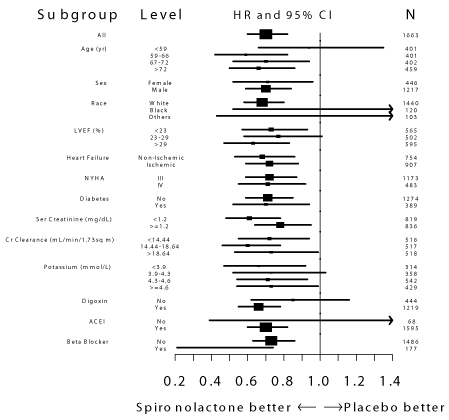
Mortality hazard ratios for some subgroups are shown in Figure 2. The favorable effect of spironolactone on mortality appeared similar for both genders and all age groups except patients younger than 55; there were too few non-whites in the Randomized Spironolactone Evaluation Study to draw any conclusions about differential effects by race. Spironolactone’s benefit appeared greater in patients with low baseline serum potassium levels and less in patients with ejection fractions <0.2. These subgroup analyses must be interpreted cautiously.
Figure 2. Hazard Ratios of All-Cause Mortality by Subgroup in The Randomized Spironolactone Evaluation Study
Figure 2: The size of each box is proportional to the sample size as well as the event rate. LVEF denotes left ventricular ejection fraction, Ser Creatinine denotes serum creatinine, Cr Clearance denotes creatinine clearance, and ACEI denotes angiotensin-converting enzyme inhibitor.
INDICATIONS AND USAGE
Spironolactone is indicated in the management of:
Primary hyperaldosteronism for:
Establishing the diagnosis of primary hyperaldosteronism by therapeutic trial.
Short-term preoperative treatment of patients with primary hyperaldosteronism.
Long-term maintenance therapy for patients with discrete aldosterone-producing adrenal adenomas who are judged to be poor operative risks or who decline surgery.
Long-term maintenance therapy for patients with bilateral micro- or macronodular adrenal hyperplasia (idiopathic hyperaldosteronism).
Edematous conditions for patients with:
Congestive heart failure: For the management of edema and sodium retention when the patient is only partially responsive to, or is intolerant of, other therapeutic measures. Spironolactone is also indicated for patients with congestive heart failure taking digitalis when other therapies are considered inappropriate.
Cirrhosis of the liver accompanied by edema and/or ascites: Aldosterone levels may be exceptionally high in this condition. Spironolactone is indicated for maintenance therapy together with bed rest and the restriction of fluid and sodium.
Nephrotic syndrome: For nephrotic patients when treatment of the underlying disease, restriction of fluid and sodium intake, and the use of other diuretics do not provide an adequate response.
Essential hypertension
Spironolactone is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Usually in combination with other drugs, spironolactone is indicated for patients who cannot be treated adequately with other agents or for whom other agents are considered inappropriate.
Hypokalemia
For the treatment of patients with hypokalemia when other measures are considered inappropriate or inadequate. Spironolactone is also indicated for the prophylaxis of hypokalemia in patients taking digitalis when other measures are considered inadequate or inappropriate.
Severe heart failure (NYHA class III - IV)
To increase survival, and to reduce the need for hospitalization for heart failure when used in addition to standard therapy.
Usage in Pregnancy.
The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developing toxemia.
Edema during pregnancy may arise from pathologic causes or from the physiologic and mechanical consequences of pregnancy.
Spironolactone is indicated in pregnancy when edema is due to pathologic causes just as it is in the absence of pregnancy (however, see PRECAUTIONS: Pregnancy). Dependent edema in pregnancy, resulting from restriction of venous return by the expanded uterus, is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is unsupported and unnecessary.
There is hypervolemia during normal pregnancy which is not harmful to either the fetus or the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort that is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
CONTRAINDICATIONS
Spironolactone is contraindicated for patients with anuria, acute renal insufficiency, significant impairment of renal excretory function, hyperkalemia, Addison’s disease, and with concomitant use of eplerenone.
WARNINGS
Potassium Supplementation.
Potassium supplementation, either in the form of medication or as a diet rich in potassium, should not ordinarily be given in association with spironolactone therapy. Excessive potassium intake may cause hyperkalemia in patients receiving spironolactone (see PRECAUTIONS: General).
Concomitant administration of spironolactone with the following drugs or potassium sources may lead to severe hyperkalemia:
- •
- other potassium-sparing diuretics
- •
- ACE inhibitors
- •
- angiotensin II antagonists
- •
- aldosterone blockers
- •
- non-steroidal anti-inflammatory drugs (NSAIDs), e.g., indomethacin
- •
- heparin and low molecular weight heparin
- •
- other drugs or conditions known to cause hyperkalemia
- •
- potassium supplements
- •
- diet rich in potassium
- •
- salt substitutes containing potassium
Spironolactone should not be administered concurrently with other potassium-sparing diuretics. Spironolactone, when used with ACE inhibitors or indomethacin, even in the presence of a diuretic, has been associated with severe hyperkalemia. Extreme caution should be exercised when spironolactone is given concomitantly with these drugs.
Hyperkalemia in Patients with Severe Heart Failure.
Hyperkalemia may be fatal. It is critical to monitor and manage serum potassium in patients with severe heart failure receiving spironolactone. Avoid using other potassium-sparing diuretics. Avoid using oral potassium supplements in patients with serum potassium > 3.5 mEq/L. The Randomized Spironolactone Evaluation Study excluded patients with a serum creatinine > 2.5 mg/dL or a recent increase in serum creatinine > 25%. The recommended monitoring for potassium and creatinine is one week after initiation or increase in dose of spironolactone, monthly for the first 3 months, then quarterly for a year, and then every 6 months. Discontinue or interrupt treatment for serum potassium > 5 mEq/L or for serum creatinine > 4 mg/dL. (See CLINICAL STUDIES: Severe Heart Failure, and DOSAGE AND ADMINISTRATION: Severe Heart Failure.)
Spironolactone should be used with caution in patients with impaired hepatic function because minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Lithium generally should not be given with diuretics (see PRECAUTIONS: Drug Interactions).
PRECAUTIONS
General.
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte imbalance, e.g., hypomagnesemia, hyponatremia, hypochloremic alkalosis, and hyperkalemia.
Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance, irrespective of cause, include dryness of the mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting. Hyperkalemia may occur in patients with impaired renal function or excessive potassium intake and can cause cardiac irregularities, which may be fatal. Consequently, no potassium supplement should ordinarily be given with spironolactone.
If hyperkalemia is suspected (warning signs include paresthesia, muscle weakness, fatigue, flaccid paralysis of the extremities, bradycardia and shock) an electrocardiogram (ECG) should be obtained. However, it is important to monitor serum potassium levels because mild hyperkalemia may not be associated with ECG changes.
If hyperkalemia is present, spironolactone should be discontinued immediately. With severe hyperkalemia, the clinical situation dictates the procedures to be employed. These may include the intravenous administration of calcium chloride solution, sodium bicarbonate solution and/or the oral or parenteral administration of glucose with a rapid-acting insulin preparation. These are temporary measures to be repeated as required. Cationic exchange resins such as sodium polystyrene sulfonate may be orally or rectally administered. Persistent hyperkalemia may require dialysis.
Reversible hyperchloremic metabolic acidosis, usually in association with hyperkalemia, has been reported to occur in some patients with decompensated hepatic cirrhosis, even in the presence of normal renal function.
Dilutional hyponatremia, manifested by dryness of the mouth, thirst, lethargy, and drowsiness, and confirmed by a low serum sodium level, may be caused or aggravated, especially when spironolactone is administered in combination with other diuretics, and dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than administration of sodium, except in rare instances when the hyponatremia is life-threatening.
Spironolactone therapy may cause a transient elevation of BUN, especially in patients with pre-existing renal impairment. Spironolactone may cause mild acidosis.
Gynecomastia may develop in association with the use of spironolactone; physicians should be alert to its possible onset. The development of gynecomastia appears to be related to both dosage level and duration of therapy and is normally reversible when spironolactone is discontinued. In rare instances some breast enlargement may persist when spironolactone is discontinued.
Somnolence and dizziness have been reported to occur in some patients. Caution is advised when driving or operating machinery until the response to initial treatment has been determined.
Information for Patients.
Patients who receive spironolactone should be advised to avoid potassium supplements and foods containing high levels of potassium including salt substitutes.
Laboratory Tests.
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done at appropriate intervals, particularly in the elderly and those with significant renal or hepatic impairments.
Drug Interactions.
ACE Inhibitors:
Concomitant administration of ACE inhibitors with potassium-sparing diuretics has been associated with severe hyperkalemia.
Angiotensin II antagonists, aldosterone blockers, heparin, low molecular weight heparin, and other drugs known to cause hyperkalemia:
Concomitant administration may lead to severe hyperkalemia.
Pressor Amines (e.g., Norepinephrine):
Spironolactone reduces the vascular responsiveness to norepinephrine. Therefore, caution should be exercised in the management of patients subjected to regional or general anesthesia while they are being treated with spironolactone.
Skeletal Muscle Relaxants, Nondepolarizing (e.g., Tubocurarine):
Possible increased responsiveness to the muscle relaxant may result.
Lithium:
Lithium generally should not be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs):
In some patients, the administration of an NSAID can reduce the diuretic, natriuretic, and antihypertensive effect of loop, potassium-sparing and thiazide diuretics. Combination of NSAIDs, e.g., indomethacin, with potassium-sparing diuretics has been associated with severe hyperkalemia. Therefore, when spironolactone and NSAIDs are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
Digoxin:
Spironolactone has been shown to increase the half-life of digoxin. This may result in increased serum digoxin levels and subsequent digitalis toxicity. It may be necessary to reduce the maintenance and digitalization doses when spironolactone is administered, and the patient should be carefully monitored to avoid over- or under-digitalization.
Drug/Laboratory Test Interactions.
Several reports of possible interference with digoxin radioimmunoassay by spironolactone, or its metabolites, have appeared in the literature. Neither the extent nor the potential clinical significance of its interference (which may be assay-specific) has been fully established.
Carcinogenesis, Mutagenesis, Impairment of Fertility.
Orally administered spironolactone has been shown to be a tumorigen in dietary administration studies performed in rats, with its proliferative effects manifested on endocrine organs and the liver. In an 18-month study using doses of about 50, 150 and 500 mg/kg/day, there were statistically significant increases in benign adenomas of the thyroid and testes and, in male rats, a dose-related increase in proliferative changes in the liver (including hepatocytomegaly and hyperplastic nodules). In a 24-month study in which the same strain of rat was administered doses of about 10, 30, 100 and 150 mg spironolactone/kg/day, the range of proliferative effects included significant increases in hepatocellular adenomas and testicular interstitial cell tumors in males, and significant increases in thyroid follicular cell adenomas and carcinomas in both sexes. There was also a statistically significant, but not dose-related, increase in benign uterine endometrial stromal polyps in females.
A dose-related (above 20 mg/kg/day) incidence of myelocytic leukemia was observed in rats fed daily doses of potassium canrenoate (a compound chemically similar to spironolactone and whose primary metabolite, canrenone, is also a major product of spironolactone in man) for a period of one year. In two-year studies in the rat, oral administration of potassium canrenoate was associated with myelocytic leukemia and hepatic, thyroid, testicular and mammary tumors.
Neither spironolactone nor potassium canrenoate produced mutagenic effects in tests using bacteria or yeast. In the absence of metabolic activation, neither spironolactone nor potassium canrenoate has been shown to be mutagenic in mammalian tests in vitro. In the presence of metabolic activation, spironolactone has been reported to be negative in some mammalian mutagenicity tests in vitro and inconclusive (but slightly positive) for mutagenicity in other mammalian tests in vitro. In the presence of metabolic activation, potassium canrenoate has been reported to test positive for mutagenicity in some mammalian tests in vitro, inconclusive in others, and negative in still others.
In a three-litter reproduction study in which female rats received dietary doses of 15 and 50 mg spironolactone/kg/day, there were no effects on mating and fertility, but there was a small increase in incidence of stillborn pups at 50 mg/kg/day. When injected into female rats (100 mg/kg/day for 7 days, i.p.), spironolactone was found to increase the length of the estrous cycle by prolonging diestrus during treatment and inducing constant diestrus during a two-week post-treatment observation period. These effects were associated with retarded ovarian follicle development and a reduction in circulating estrogen levels, which would be expected to impair mating, fertility and fecundity. Spironolactone (100 mg/kg/day), administered i.p. to female mice during a two-week cohabitation period with untreated males, decreased the number of mated mice that conceived (effect shown to be caused by an inhibition of ovulation) and decreased the number of implanted embryos in those that became pregnant (effect shown to be caused by an inhibition of implantation), and at 200 mg/kg, also increased the latency period to mating.
Teratogenic Effects.
Pregnancy Category C.
Teratology studies with spironolactone have been carried out in mice and rabbits at doses of up to 20 mg/kg/day. On a body surface area basis, this dose in the mouse is substantially below the maximum recommended human dose and, in the rabbit, approximates the maximum recommended human dose. No teratogenic or other embryotoxic effects were observed in mice, but the 20 mg/kg dose caused an increased rate of resorption and a lower number of live fetuses in rabbits. Because of its anti-androgenic activity and the requirement of testosterone for male morphogenesis, spironolactone may have the potential for adversely affecting sex differentiation of the male during embryogenesis. When administered to rats at 200 mg/kg/day between gestation days 13 and 21 (late embryogenesis and fetal development), feminization of male fetuses was observed. Offspring exposed during late pregnancy to 50 and 100 mg/kg/day doses of spironolactone exhibited changes in the reproductive tract including dose-dependent decreases in weights of the ventral prostate and seminal vesicle in males, ovaries and uteri that were enlarged in females, and other indications of endocrine dysfunction, that persisted into adulthood. There are no adequate and well-controlled studies with spironolactone in pregnant women. Spironolactone has known endocrine effects in animals including progestational and antiandrogenic effects. The antiandrogenic effects can result in apparent estrogenic side effects in humans, such as gynecomastia. Therefore, the use of spironolactone in pregnant women requires that the anticipated benefit be weighed against the possible hazards to the fetus.
Nursing Mothers.
Canrenone, a major (and active) metabolite of spironolactone, appears in human breast milk. Because spironolactone has been found to be tumorigenic in rats, a decision should be made whether to discontinue the drug, taking into account the importance of the drug to the mother. If use of the drug is deemed essential, an alternative method of infant feeding should be instituted.
ADVERSE REACTIONS
The following adverse reactions have been reported and, within each category (body system), are listed in order of decreasing severity.
Digestive: Gastric bleeding, ulceration, gastritis, diarrhea and cramping, nausea, vomiting.
Reproductive: Gynecomastia (see PRECAUTIONS), inability to achieve or maintain erection, irregular menses or amenorrhea, postmenopausal bleeding, breast pain. Carcinoma of the breast has been reported in patients taking spironolactone but a cause and effect relationship has not been established.
Hematologic: Leukopenia (including agranulocytosis), thrombocytopenia.
Hypersensitivity: Fever, urticaria, maculopapular or erythematous cutaneous eruptions, anaphylactic reactions, vasculitis.
Metabolism: Hyperkalemia, electrolyte disturbances (see WARNINGS and PRECAUTIONS).
Musculoskeletal: Leg cramps.
Nervous System/Psychiatric: Lethargy, mental confusion, ataxia, dizziness, headache, drowsiness.
Liver/Biliary: A very few cases of mixed cholestatic/hepatocellular toxicity, with one reported fatality, have been reported with spironolactone administration.
Renal: Renal dysfunction (including renal failure).
Skin: Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), drug rash with eosinophilia and systemic symptoms (DRESS), alopecia, pruritis.
OVERDOSAGE
The oral LD50 of spironolactone is greater than 1,000 mg/kg in mice, rats, and rabbits.
Acute overdosage of spironolactone may be manifested by drowsiness, mental confusion, maculopapular or erythematous rash, nausea, vomiting, dizziness, or diarrhea. Rarely, instances of hyponatremia, hyperkalemia, or hepatic coma may occur in patients with severe liver disease, but these are unlikely due to acute overdosage. Hyperkalemia may occur, especially in patients with impaired renal function.
Treatment.
Induce vomiting or evacuate the stomach by lavage. There is no specific antidote. Treatment is supportive to maintain hydration, electrolyte balance, and vital functions.
Patients who have renal impairment may develop spironolactone-induced hyperkalemia. In such cases, spironolactone should be discontinued immediately. With severe hyperkalemia, the clinical situation dictates the procedures to be employed. These may include the intravenous administration of calcium chloride solution, sodium bicarbonate solution and/or the oral or parenteral administration of glucose with a rapid-acting insulin preparation. These are temporary measures to be repeated as required. Cationic exchange resins such as sodium polystyrene sulfonate may be orally or rectally administered. Persistent hyperkalemia may require dialysis.
DOSAGE AND ADMINISTRATION
Primary Hyperaldosteronism.
Spironolactone may be employed as an initial diagnostic measure to provide presumptive evidence of primary hyperaldosteronism while patients are on normal diets.
Long Test: Spironolactone is administered at a daily dosage of 400 mg for three to four weeks. Correction of hypokalemia and of hypertension provides presumptive evidence for the diagnosis of primary hyperaldosteronism.
Short Test: Spironolactone is administered at a daily dosage of 400 mg for four days. If serum potassium increases during spironolactone administration but drops when spironolactone is discontinued, a presumptive diagnosis of primary hyperaldosteronism should be considered.
After the diagnosis of hyperaldosteronism has been established by more definitive testing procedures, spironolactone may be administered in doses of 100 to 400 mg daily in preparation for surgery. For patients who are considered unsuitable for surgery, spironolactone may be employed for long-term maintenance therapy at the lowest effective dosage determined for the individual patient.
Edema in Adults (Congestive Heart Failure, Hepatic Cirrhosis, or Nephrotic Syndrome).
An initial daily dosage of 100 mg of spironolactone administered in either single or divided doses is recommended, but may range from 25 to 200 mg daily. When given as the sole agent for diuresis, spironolactone should be continued for at least five days at the initial dosage level, after which it may be adjusted to the optimal therapeutic or maintenance level administered in either single or divided daily doses. If, after five days, an adequate diuretic response to spironolactone has not occurred, a second diuretic that acts more proximally in the renal tubule may be added to the regimen. Because of the additive effect of spironolactone when administered concurrently with such diuretics, an enhanced diuresis usually begins on the first day of combined treatment; combined therapy is indicated when more rapid diuresis is desired. The dosage of spironolactone should remain unchanged when other diuretic therapy is added.
Essential Hypertension.
For adults, an initial daily dosage of 50 to 100 mg of spironolactone administered in either single or divided doses is recommended. Spironolactone may also be given with diuretics that act more proximally in the renal tubule or with other antihypertensive agents. Treatment with spironolactone should be continued for at least two weeks, since the maximum response may not occur before this time. Subsequently, dosage should be adjusted according to the response of the patient.
Hypokalemia.
Spironolactone in a dosage ranging from 25 mg to 100 mg daily is useful in treating a diuretic-induced hypokalemia, when oral potassium supplements or other potassium-sparing regimens are considered inappropriate.
Severe heart failure in conjunction with standard therapy (NYHA class III – IV).
Treatment should be initiated with spironolactone 25 mg once daily if the patient’s serum potassium is ≤5.0 mEq/L and the patient’s serum creatinine is ≤ 2.5 mg/dL. Patients who tolerate 25 mg once daily may have their dosage increased to 50 mg once daily as clinically indicated. Patients who do not tolerate 25 mg once daily may have their dosage reduced to 25 mg every other day. See WARNINGS: Hyperkalemia in Patients with Severe Heart Failure for advice on monitoring serum potassium and serum creatinine.
HOW SUPPLIED
Spironolactone Tablets, USP are available as:
25 mg: white, round, film-coated tablets, debossed "5880" on one side and debossed "V" on the reverse side, supplied as follows:
- •
- Bottles of 30: NDC 63187-634-30
- •
- Bottles of 60: NDC 63187-634-60
- •
- Bottles of 90: NDC 63187-634-90