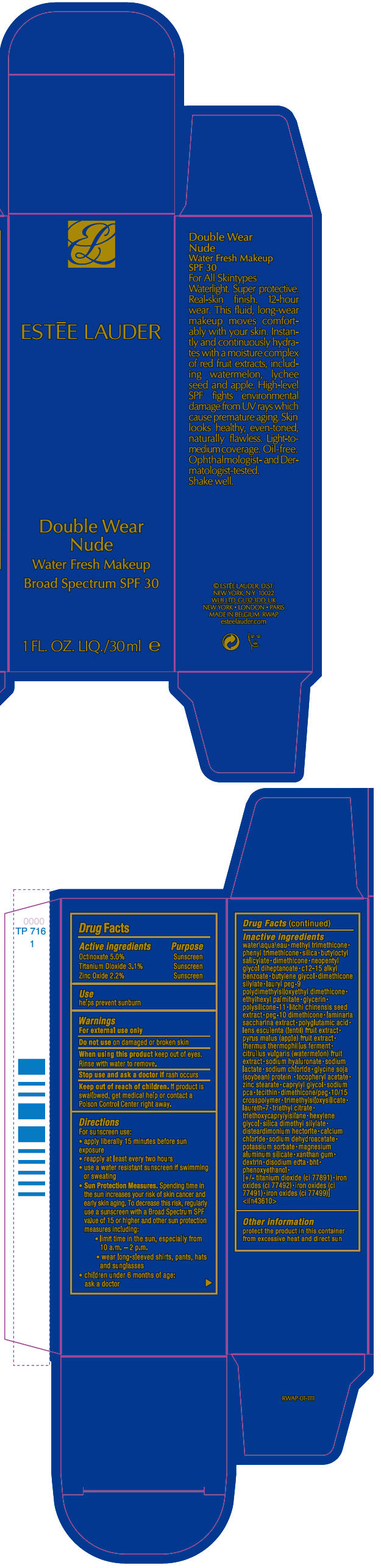
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
Inactive ingredients
water\aqua\eau • methyl trimethicone • phenyl trimethicone • silica • butyloctyl salicylate • dimethicone • neopentyl glycol diheptanoate • c12-15 alkyl benzoate • butylene glycol • dimethicone silylate • lauryl peg-9 polydimethylsiloxyethyl dimethicone • ethylhexyl palmitate • glycerin • polysilicone-11 • litchi chinensis seed extract • peg-10 dimethicone • laminaria saccharina extract • polyglutamic acid • lens esculenta (lentil) fruit extract • pyrus malus (apple) fruit extract • thermus thermophillus ferment • citrullus vulgaris (watermelon) fruit extract • sodium hyaluronate • sodium lactate • sodium chloride • glycine soja (soybean) protein • tocopheryl acetate • zinc stearate • caprylyl glycol • sodium pca • lecithin • dimethicone/peg-10/15 crosspolymer • trimethylsiloxysilicate • laureth-7 • triethyl citrate • triethoxycaprylylsilane • hexylene glycol • silica dimethyl silylate • disteardimonium hectorite • calcium chloride • sodium dehydroacetate • potassium sorbate • magnesium aluminum silicate • xanthan gum • dextrin • disodium edta • bht • phenoxyethanol • [+/- titanium dioxide (ci 77891) • iron oxides (ci 77492) • iron oxides (ci 77491) • iron oxides (ci 77499)] <iln43610>