Uses temporarily ■ reduces fever
■ relieves minor aches and pains due to:
■ the common cold ■ flu ■ headache ■ sore throat
■ toothache
Warnings
Liver warning
This product contains acetaminophen. Severe liver
damage may occur if your child takes: ■ more than 5
doses in 24 hours, which is the maximum daily amount
■ with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin
reactions. Symptoms may include: ■ skin reddening
■ blisters ■rash
If a skin reaction occurs, stop use and seek medical
help right away.
Sore throat warning
If sore throat is severe, persists for more than 2 days,
is accompanied or followed by fever, headache, rash,
Do not use
■ with any other drug containing acetaminophen
(prescription or nonprescription). If you are not sure
whether a drug contains acetaminophen, ask a doctor
or pharmacist.
■ if your child is allergic to acetaminophen or any of
the inactive ingredients in this product.
Stop use and ask a doctor if
■ pain gets worse or lasts more than 5 days ■ fever
gets worse or lasts more than 3 days ■ new symptoms occur ■ redness or swelling is present
These could be signs of a serious condition.
Overdose warning
Taking more than the recommended dose (overdose)
may cause liver damage. In case of overdose, get
medical help or contact a Poison Control Center right
away. (1-800-222-1222) Quick medical attention
is critical for adults as well as for children even if you
do not notice any signs or symptoms.
Directions
■ this product does not contain directions or
complete warnings for adult use.
■ shake well before using
■ mL = milliliter; tsp = teaspoonful
■ find right dose on chart below. If possible, use
weight to dose; otherwise, use age.
■ if needed, repeat dose every 4 hours while
symptoms last
■ do not give more than 5 times in 24 hours
■ do not give for more than 5 days unless directed
by adoctor.
Weight (Ib) Age (yr) Dose (mL or tsp)*
under 24 under 2 years ask a doctor
24-35 2-3 years 5 mL (1 tsp)
36-47 4-5 years 7.5 mL (1 Y2 tsp)
48-59 6-8 years 10 mL (2 tsp)
60-71 9-10 year 12.5 mL (2Y2 tsp)
72-95 11 year 15 mL (3 tsp)
*or as directed by a doctor
Attention: use only enclosed dosing cup specifically
designed for use with this product. Do not use any
other dosing device.
Other information
■ each 5 mL (1 tsp) contains: sodium 3 mg
■ store between 20-25°C (68-77oF)
■ do not refrigerate
■ Keep carton for full directions for use.
Inactive ingredients anhydrous citric acid,
butylparaben, FD&C red #40, flavor, glycerin, high
fructose corn syrup, microcrystalline cellulose and
carboxymethylcellulose sodium, propylene glycol,
purified water, sodium benzoate, sorbitol solution,
sucralose, xanthan gum
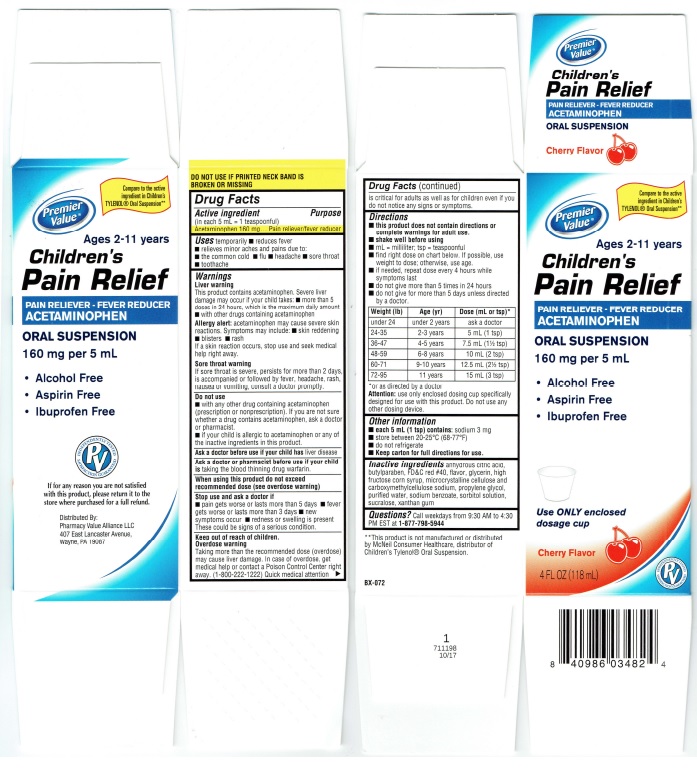
Principal Display Panel
Premier
Value®
Children's
Pain Relief
PAIN RELIEVER – FEVER REDUCER
ACETAMINOPHEN
ORAL SUSPENSION
Cherry Flavor
Premier
Value®
Compare to the active
ingredients in Children's
TYLENOL® Oral Suspension**
Ages 2-11 years
Children's
Pain Relief
PAIN RELIEVER – FEVER REDUCER
ACETAMINOPHEN
ORAL SUSPENSION
160 mg per 5 mL
• Alcohol Free
• Aspirin Free
• Ibuprofen Free
Use ONLY enclosed
dosage cup
Cherry Flavor
4 FL OX (118 mL)
INDEPENDENTLY TESTED
PV
SATISFACTION GUARANTEED
DO NOT USE IF PRINTED NECK BAND IS
BROKEN OR MISSING
If for any reason you are not satisfied
with this product, please return it to the
store where purchased for a full refund.
Distributed By:
Pharmacy Value Alliance LLC
407 East Lancaster Avenue,
Wayne, PA 19087
**This product is not manufactured or distributed
by McNeil Consumer Healthcare, distributor of
Children's Tyleno!® Oral Suspension.
BX-072
711198
10/17
Product Label

Premier Value® Children's Pain Relief ACETAMINOPHEN ORAL SUSPENSION Cherry Flavor


end res