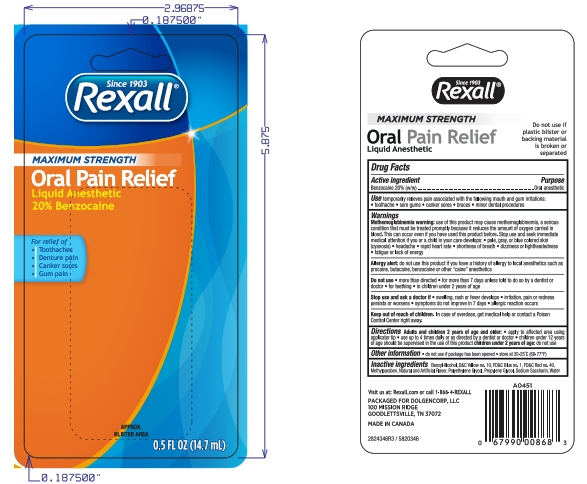
Uses
Temporarily relieves pain associated with the following mouth irritations toothache sore gums canker sores braces minor dental procedures
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
When using this product
- avoid contact with eyes
- do not exceed recommended dosage
- Do not use more than directed for more than 7 days unless directed by a dentist or doctor.
Stop use and ask a doctor if sore mouth symptoms do not improve in 7 days, swelling, rash or fever develops, irritation, pain or redness persists or worsens swelling rash or fever developes
Directions
Adults and children 2 years of age and older:
- apply to affect4ed area using applicator tip
- use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age should be supervised in the use of this product.
Children under 2 years of age: ask a dentist or doctor.
Inactive ingredients
Benzyl Alcohol, D&C Yellow 10, FD&C Blue 1, FD&C Red 40, FD&C Yellow 5, Methylparaben, Flavor, Polyethylene Glycol, Propylene Glycol, Sodium Saccharin, Water