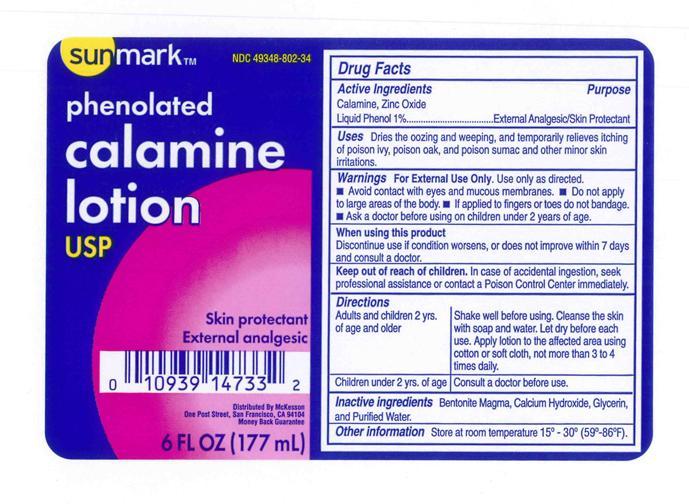
Uses
Dries the oozing and weeping and temporarily pain and itching of poison ivy, poison oak, and poison sumac, or other minor skin irritations
Warnings
- For external use only. Use only as directed.
- Avoid contact with eyes and mucous membranes.
- Do not apply to large areas of the body or in large quantities, particularly over raw or blistered areas.
- If applied to fingers or toes do not bandage.
Ask a doctor
before using on children under 2 years of age.
When using this product. Discontinue use if condition worsen or does not improve within 7 days and consult a doctor.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a poison Control Center immediately.
directions (Shake well before using)
Adult and children 2 years of age and older: Cleanse the skin with soap and water and let dry before each use. Apply product to the affected area using cotton or soft cloth, as often as needed for comfort.
Children under 2 years of age: Consult a doctor before use.