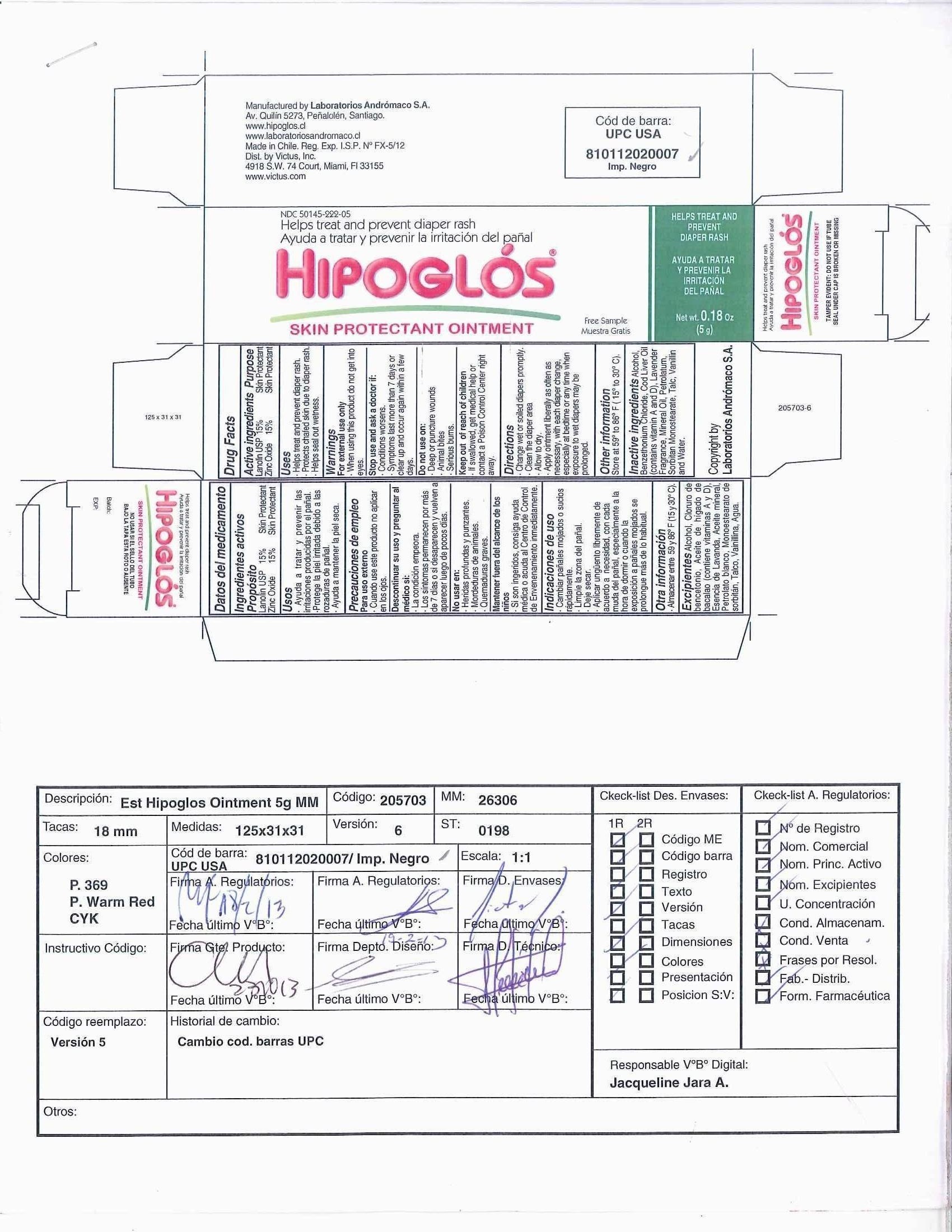
Uses
- Helps treat and prevent diaper rash.
- Protects chafed skin due to diaper rash.
- Helps seal out wetness.
Warnings
For external use only
When using this product - do not get into eyes
Stop use and ask a doctor if:
- Conditions worsen.
- Symptoms last more than 7 days or clear up and occur again within a few days.
Do not use on:
- Deep or puncture wounds
- Animal Bites
- Serious Burns
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Change wet or soiled diapers promptly.
- Clean the diaper area.
- Allow to dry.
- Apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or any time when exposure to wet diapers may be prolonged.
Inactive ingredients
Alcohol, Benzethonium Chloride, Cod Liver Oil (contains vitamin A and D), Lavender Fragrance, Mineral Oil, Petrolatum, Sorbitan Monostearate, Talc, Vanillin, and Water.
Usos
- Ayuda a tratar y prevenir las irritaciones producidas por el pañal.
- Protege la piel irritada debido a las rozaduras de pañal.
- Ayuda a mantener la piel seca.
Warnings
Para uso externo
*Cuando use este producto no aplicar en los ojos.
Descontinuar su uso y preguntar al médico si:
• la condición empeora
• Los síntomas permanecen por más de 7 días o si desaparecen y vuelven a aparecer luego de pocos días.
No usar en:
• Heridas profundas y punzantes.
• Mordeduras de animales
• Quemaduras graves
Mantener fuera del alcance de los niños
* Si son ingeridos, consiga ayuda médica o acuda al Centro de Control de Envenenamiento inmediatamente.
Indicaciones de uso
- Cambiar pañales mojados o sucios rápidamente.
- Limpie la zona del pañal.
- Deje secar.
- Aplicar ungüento libremente de acuerdo a necesidad, con cada muda del pañal, especialmente a la hora de dormir o cuando la exposición a pañales mojados se prolongue más de lo habitual.
Excipientes
alcohol, cloruro de bencetonio, aceite de hígado de bacalao (contiene vitaminas A y D), esencia de lavanda, aceite mineral, petrolato blanco, monoestearato de sorbitan, talco, vainillina, agua.