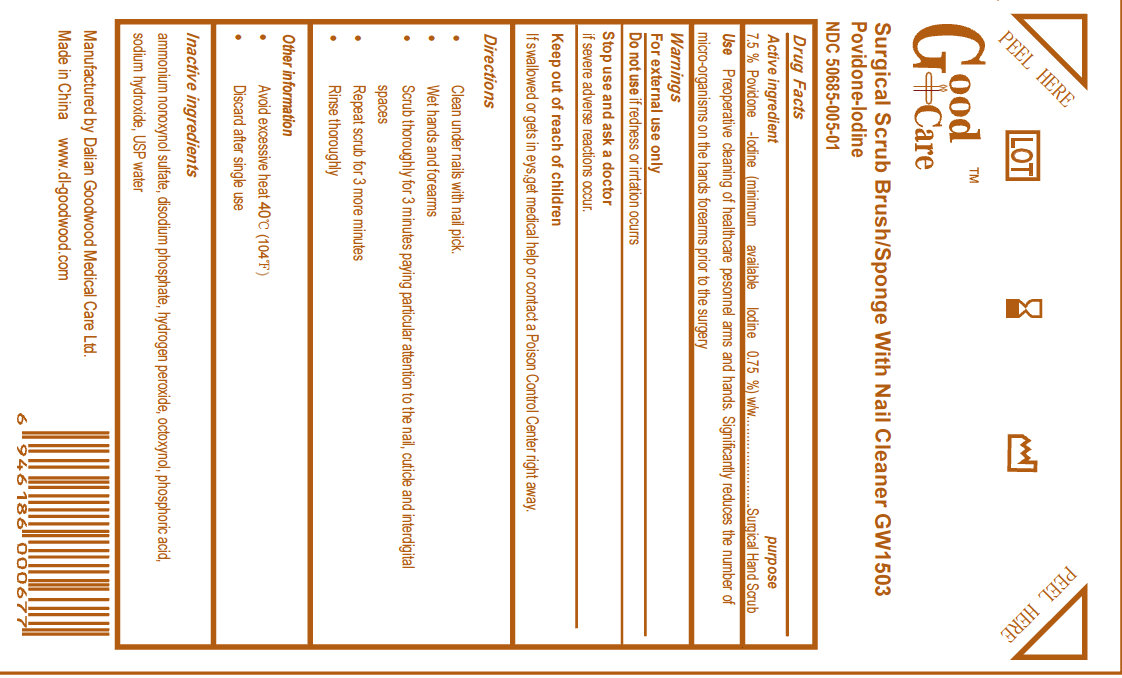
GOOD CARE SURGICAL BRUSH WITH NAIL CLEANER- povidone-iodine sponge
Dalian Goodwood Medical Care Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Povidone Iodine USP, 7.5% w/v
(minimum available Iodine 0.75%) w/w
Purpose
Surgical Hand Scrub
Use
Preoperative cleaning of healthcare personnel arms and hands. Significantly reduces the number of micro-organisms on the hands forearms prior to the surgery.
Warnings
For external use only.
Do not use if redness or irritation occur
Stop use and ask a doctor if severe adverse reactions occur.
Keep out of reach of children. If swallowed or gets in eyes, get medical help or contact a Poison Control Center right away.
Directions
- Clean under nails with nail pick
- Wet hands and forearms
- Scrub thoroughly for 3 minutes paying particular attention to the nail, cuticle and interdigital spaces
- Repeat scrub for 3 more minutes
- Rinse thoroughly
Other information
- Avoid excessive heat 40 degrees C (104 degrees F)
- Discard after single use
Inactive ingredients
ammonium nonoxynol sulfate, disodium phosphate, hydrogen peroxide, octoxynol, phosphoric acid, sodium hydroxide, USP water
Manufactured by DALIAN GOODWOOD MEDICAL CARE LTD.
Made in China www.dl-goodwood.com
Good Care
Surgical Scrub Brush/ Sponge With Nail Cleaner GW1503
Povidone-Iodine
Dalian Goodwood Medical Care Ltd.