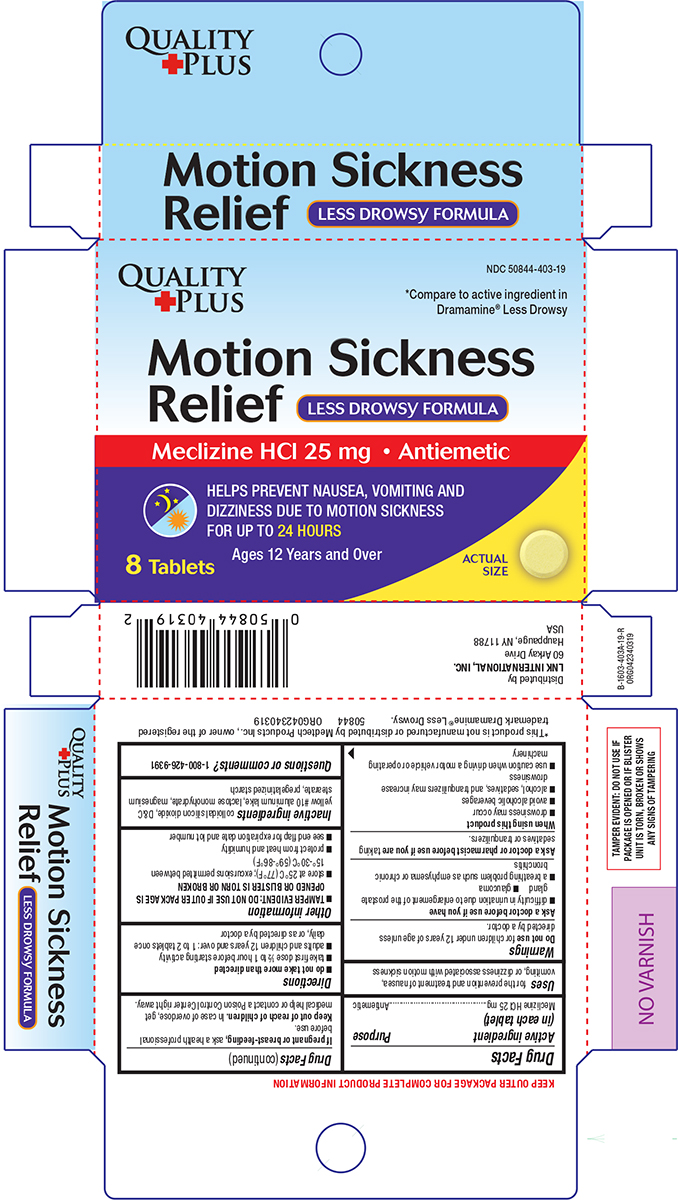
Uses
for the prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness
Warnings
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
Directions
- do not take more than directed
- take first dose ½ to 1 hour before starting activity
- adults and children 12 years and over: 1 to 2 tablets once daily, or as directed by a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from heat and humidity
- see end flap for expiration date and lot number
Inactive ingredients
colloidal silicon dioxide, D&C yellow #10 aluminum lake, lactose monohydrate, magnesium stearate, pregelatinized starch
Principal Display Panel
QUALITY
+PLUS
NDC 50844-403-19
*Compare to active ingredient in
Dramamine® Less Drowsy
Motion Sickness
Relief
LESS DROWSY FORMULA
Meclizine HCl 25 mg • Antiemetic
HELPS PREVENT NAUSEA, VOMITING AND
DIZZINESS DUE TO MOTION SICKNESS
FOR UP TO 24 HOURS
Ages 12 Years and Over
8Tablets
ACTUAL
SIZE
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Medtech Products Inc., owner of the registered trademark
Dramamine® All Day Less Drowsy. 50844 ORG042340319
Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA
Quality Plus 44-403A