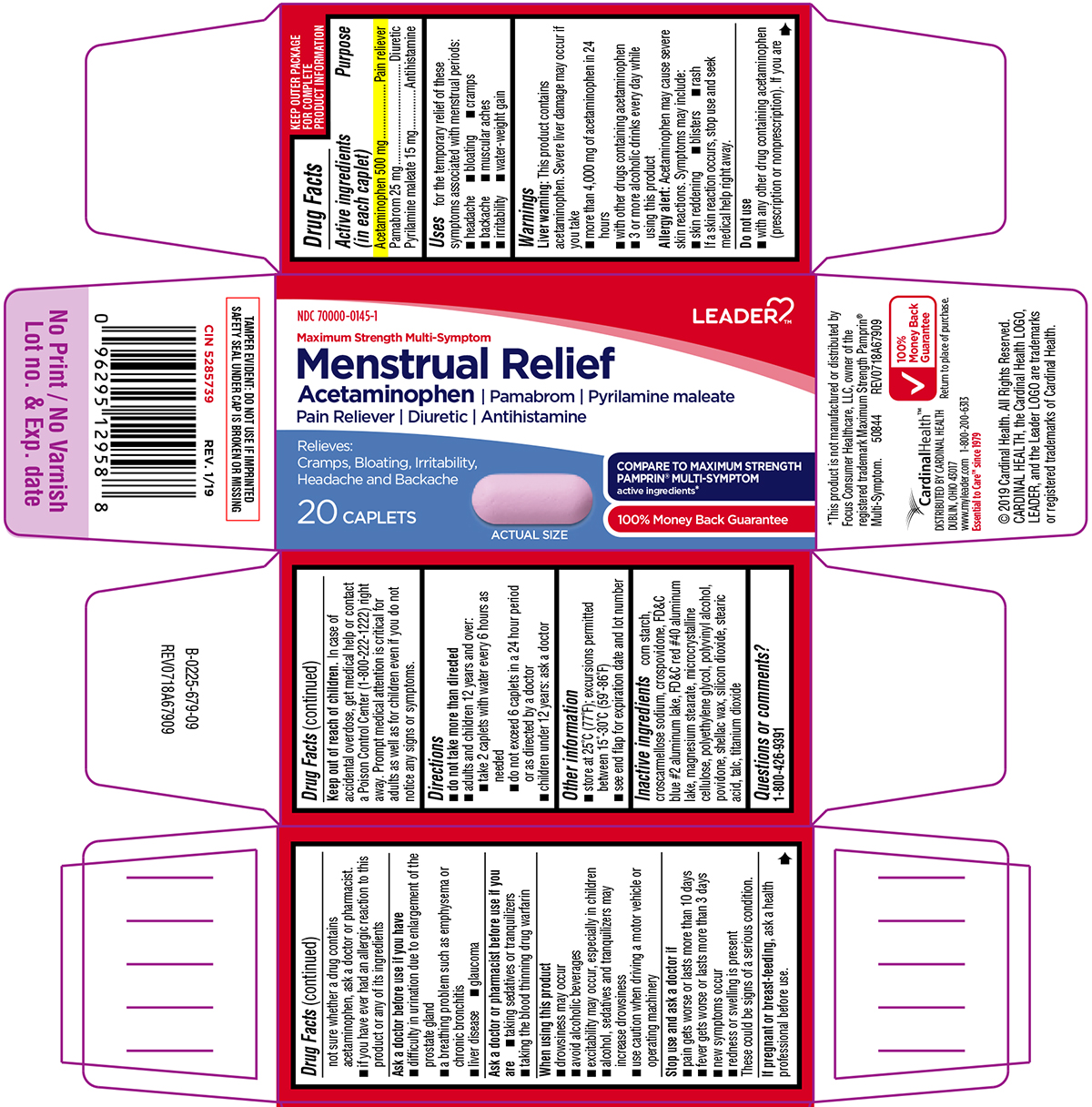
Uses
for the temporary relief of these symptoms associated with menstrual periods:
- headache
- bloating
- cramps
- backache
- muscular aches
- irritability
- water-weight gain
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness may occur
- avoid alcoholic beverages
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
Directions
-
do not take more than directed
- adults and children 12 years and over:
- take 2 caplets with water every 6 hours as needed
- do not exceed 6 caplets in a 24 hour period or as directed by a doctor
- children under 12 years: ask a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, croscarmellose sodium, crospovidone, FD&C blue #2 aluminum lake, FD&C red #40 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, shellac wax, silicon dioxide, stearic acid, talc, titanium dioxide
Principal Display Panel
LEADER™
NDC 70000-0145-1
Maximum Strength Multi-Symptom
Menstrual Relief
Acetaminophen | Pamabrom | Pyrilamine maleate
Pain Reliever | Diuretic | Antihistamine
Relieves:
Cramps, Bloating, Irritability,
Headache and Backache
20 CAPLETS
ACTUAL SIZE
COMPARE TO MAXIMUM STRENGTH PAMPRIN® MULTI-SYMPTOM active ingredients*
100% Money Back Guarantee
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Focus Consumer Healthcare, LLC, owner of the registered trademark Maximum Strength Pamprin® Multi-Symptom.
50844 REV0718A67909
CardinalHealth™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
Essential to Care™ since 1979
© 2019 Cardinal Health. All Rights Reserved.
CARDINAL HEALTH, the Cardinal Health LOGO,
LEADER, and the Leader LOGO are trademarks
or registered trademarks of Cardinal Health.
100% Money Back Guarantee
Return to place of purchase.
Leader 44-679