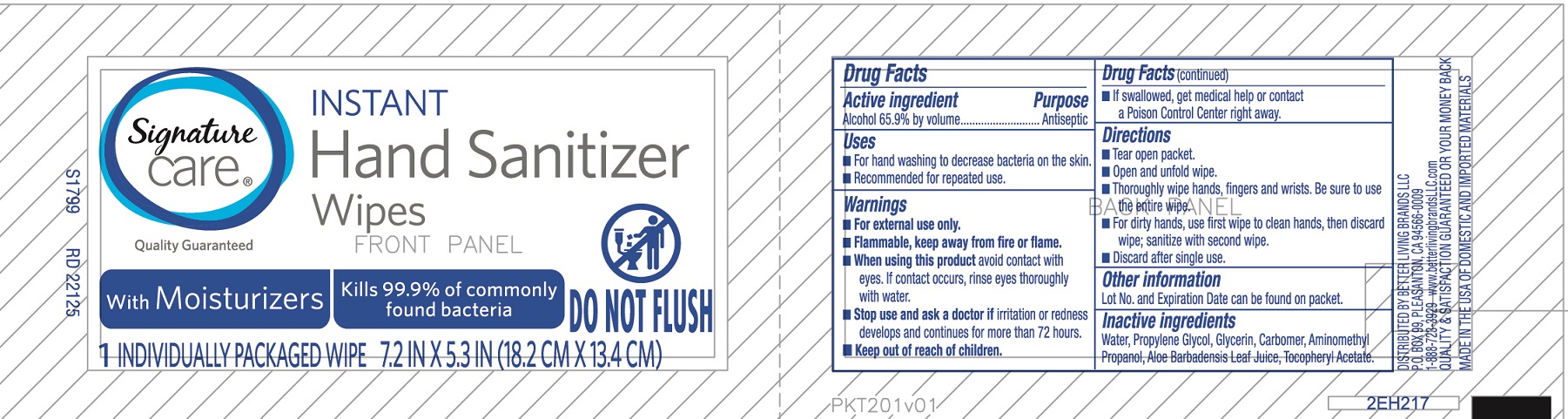
SAFEWAY INSTANT HAND SANITIZING WIPES- alcohol cloth
Safeway Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Alcohol 65.9% by volume
Uses
- For handwashing to decrease bacteria on the skin.
- Recommended for repeated use.
Warnings
-
For external use only.
-
Flammable, keep away from fire or flame.
When using this product
avoid contact with eyes, If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
irritation or redness develops and continues for more than 72 hours.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Open and unfold wipe.
- Thoroughly wipe hands, fingers and wrists. Be sure to use the entire wipe.
- For dirty hands, use first wipe to clean hands, then discard wipe; sanitize with second wipe.
- Discard after single use.
Other information
Lot No. and Expiration Date can be found on packet.
Dosage
For dirty hands, use first wipe to clean hands, then discard wipe. Sanitize with a second wipe. Discard after single use.
Inactive ingredients
Water, Propylene Glycol, Glycerin, Carbomer, Aminomethyl Propanol, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate
Principal Display Panel
Signature Care
Instant Hand Sanitizer Wipes
With Moisturizers
Kills 99.9% of commonly found bacteria
24 Individually PAckaged Wipes
Do Not Flush
1 individually packaged wipe
7.2 in x 5.3 in (18.2 cm x 13.4 cm)
Packet label:
Box label:

Safeway Inc.

