Uses
- temporarily relieves cough due to minor throat and bronchial irritation associated with the common cold
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not exceed 6 doses in 24 hours
- take with a full glass of water
- adults and children 12 years of age and older
- take 1 tablet every 4 hours
- children under 12 years of age: do not use
Inactive ingredients
colloidal silicon dioxide, magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
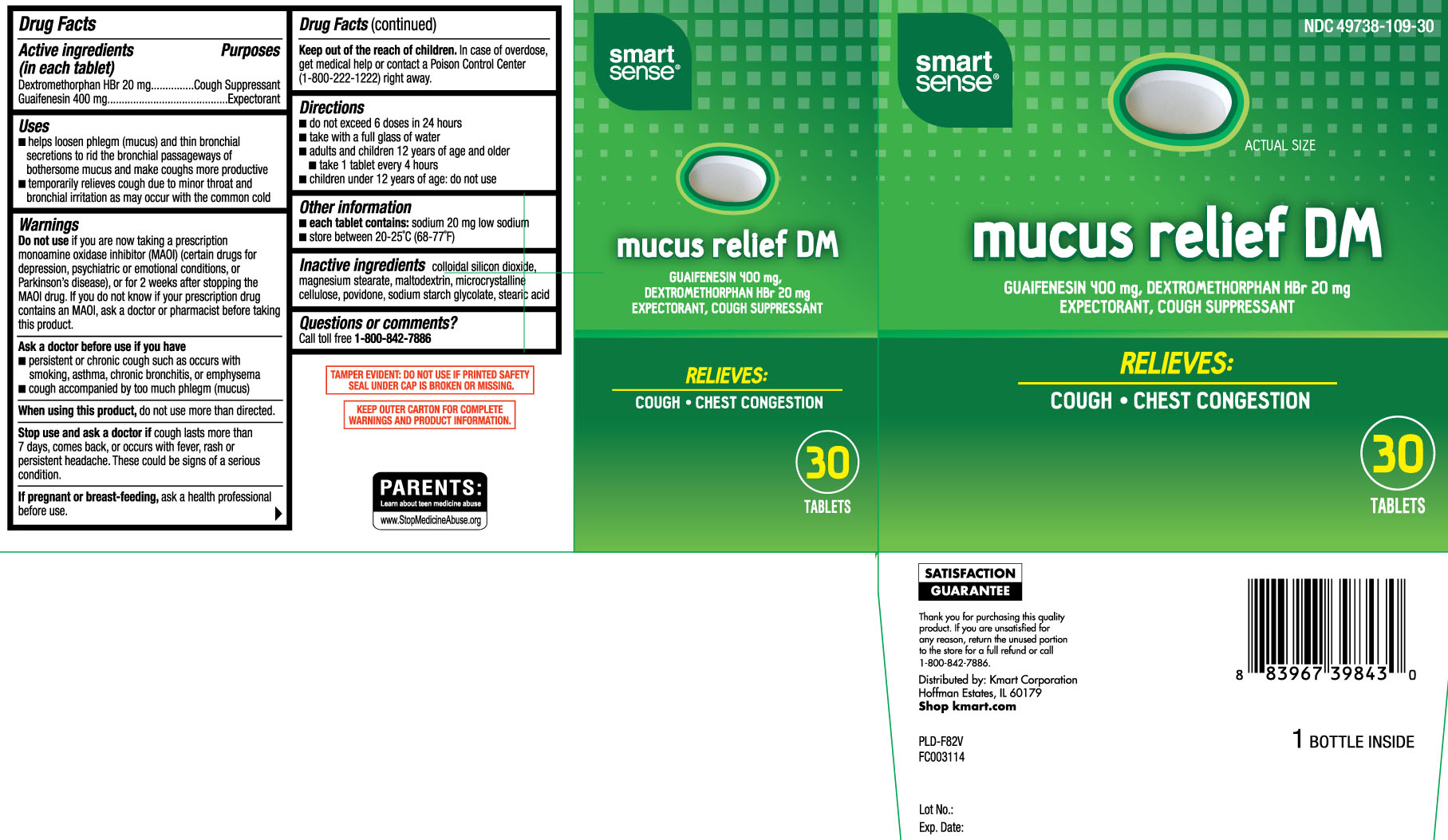
Principal Display Panel
Mucus relief DM
GUAIFENESIN 400 mg, DEXTROMETHORPHAN HBr 20 mg
EXPECTORANT, COUGH SUPPRESSANT
RELIEVES:
COUGH • CHEST CONGESTION
TABLETS
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: Kmart Corporation
Hoffman Estates, IL 60179
Shop kmart.com