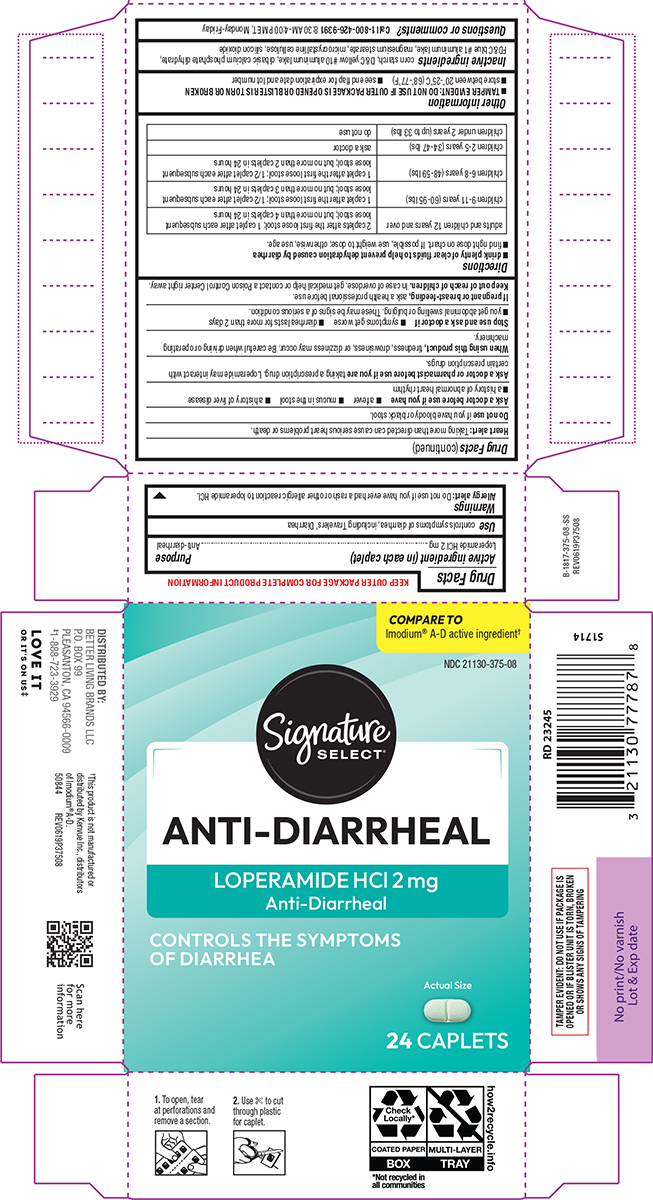
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.
Ask a doctor before use if you have
- a fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children 2-5 years (34-47 lbs) | ask a doctor |
| children under 2 years (up to 33 lbs) | do not use |
Other information
-
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store between 20º-25ºC (68º-77ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide
Principal Display Panel
COMPARE TO
Imodium® A-D active ingredient†
Signature
SELECT®
NDC 21130-375-08
Anti-Diarrheal
LOPERAMIDE HCl 2 mg
Anti-Diarrheal
Controls the symptoms
of diarrhea
Actual Size
24 CAPLETS
† This product is not manufactured or
distributed by Kenvue Inc., distributors
of Imodium® A-D.
50844 REV0619P37508
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING
DISTRIBUTED BY:
BETTER LIVING BRANDS LLC
P.O. BOX 99
PLEASANTON, CA 94566-0009
‡1-888-723-3929
1. To open, tear
at perforations and
remove a section.
2. Use ✄ to cut
through plastic
for caplet.
LOVE IT
OR IT'S ON US‡
Signature Care 44-375