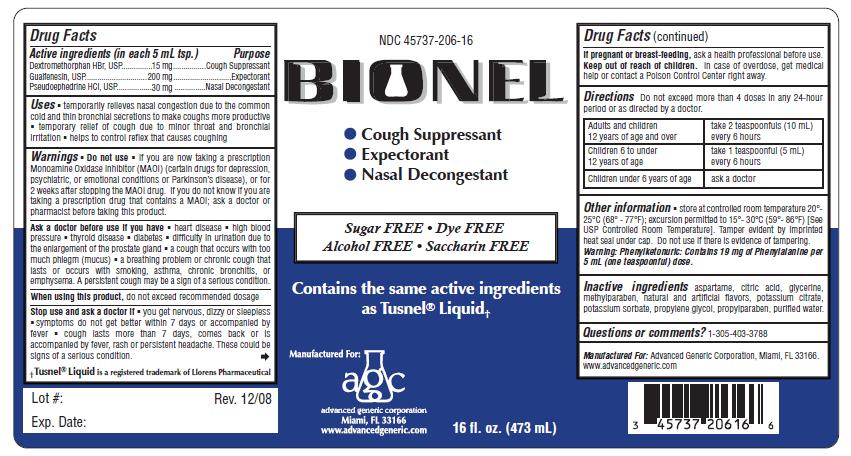
Active Ingredients: (in each 5 mL tsp.) Purpose
Guaifenesin 200 mg .............................................. Expectorant
Dextromethorphan Hydrobromide 15 mg.................. Cough Suppressant
Pseudoephedrine HCl 30 mg.................................. Nasal Decongestant
- temporarily relieves nasal congestion due to the common cold and thin bronchial secretions to make coughs more productive
- temporary relief of cough due to minor throat and bronchial irritation
- helps to control reflex that causes coughing
Warnings
Do not use if you are now taking a prescription
Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression,
psychiatric, or emotional conditions or Parkinson’s disease), or for
2 weeks after stopping the MAOI drug. If you do not know if you are
taking a prescription drug that contains a MAOI; ask a doctor or
pharmacist before taking this product.
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to the enlargement of the prostate gland
- a cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or occurs with smoking, asthma, chronic bronchitis, or emphysema. A persistent cough may be a sign of a serious condition.
- you get nervous, dizzy or sleepless
- symptoms do not get better within 7 days or accompanied by fever
- cough lasts more than 7 days, comes back or is accompanied by fever, rash or persistent headache. These could be signs of a serious condition.
Directions Do not exceed 4 doses in 24 hours.
adults and children 12 years of age and over take 2 teaspoonful (10 mL) every 6 hours
children 6 to under 12 years of age take 1 teaspoonful (5 mL) every 4 hours
children under 6 years of age ask a doctor
Other information
store at controlled room temperature 20°- 25°C (68° - 77°F); excursion permitted to 15°- 30°C (59°- 86°F) [See USP Controlled Room Temperature]. Tamper evident by imprinted heat seal under cap. Do not use if there is evidence of tampering.
Warning: Phenylketonuric: Contains 19 mg of Phenylalanine per 5 mL (one teaspoonful) dose.
Inactive ingredients aspartame, citric acid, glycerine, methylparaben, natural and artificial flavors, potassium citrate, potassium sorbate, propylene glycol, propylparaben, purified water