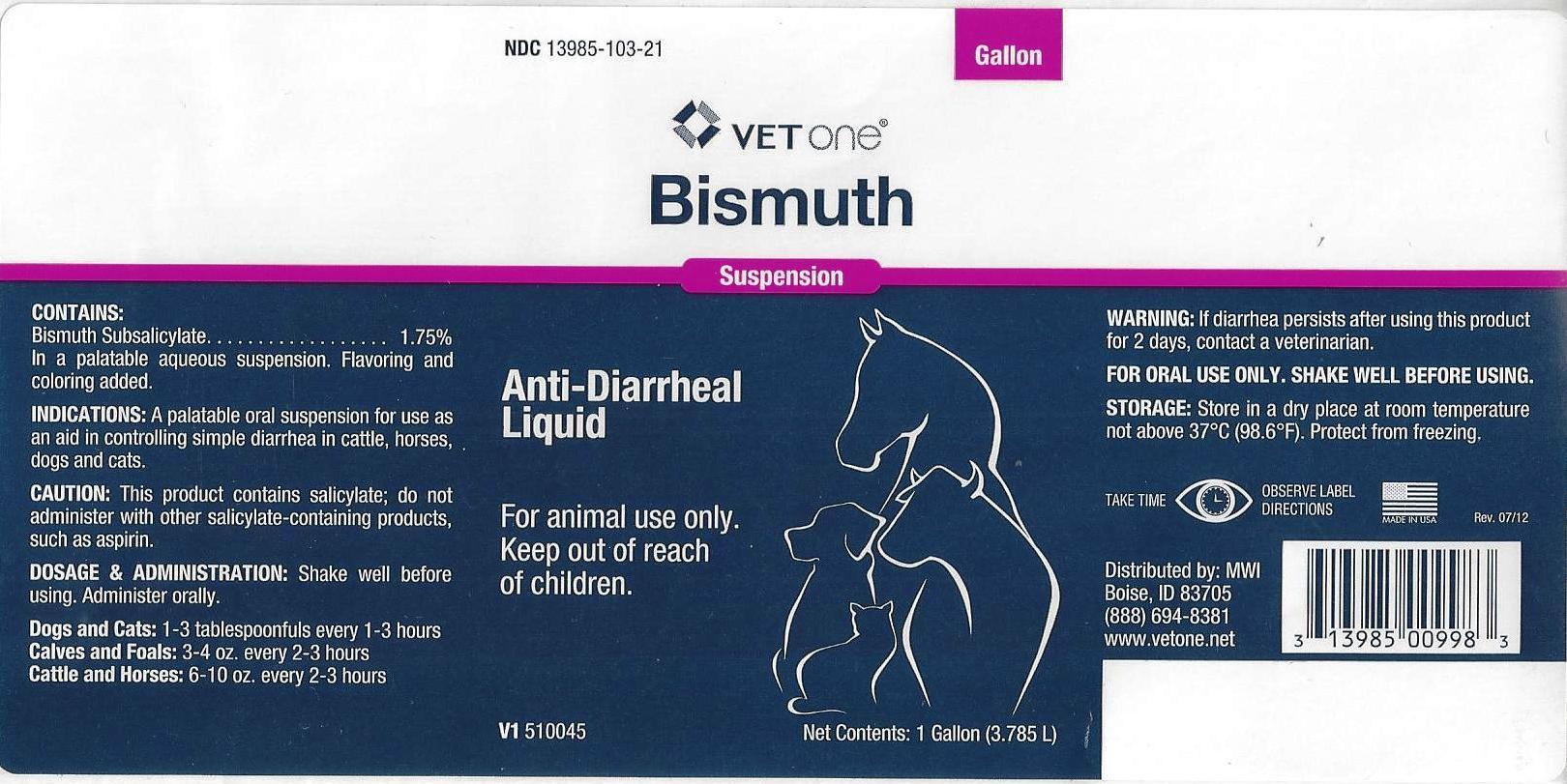
NDC 13985-103-21
VET one
Bismuth
Suspension
Anti-Diarrheal Liquid
For animal use only.
Keep out of reach of children.
V1 510045
Net Contents: 1 Gallon (3.785L)
CONTAINS:
Bismuth Subsalicylate ... 1.75%
In a palatable aqueous suspension. Flavoring and coloring added.
INDICATIONS:
A palatable oral suspension for use as an aid in controlling simple diarrhea in cattle, horses, dogs and cats
CAUTION:
This product contains salicylate; do not administer with other salicylate-containing products, such as aspirin.
DOSAGE & ADMINISTRATION:
Shake well before using. Administer orally.
Dogs and Cats: 1 to 3 tablespoonfuls every 1-3 hours
Calves and Foals: 3 to 4 oz. every 2-3 hours
Cattle and Horses: 6 to 10 oz. every 2-3 hours
WARNING:
If diarrhea persists after using this product for 2 days, contact a veterinarian.
FOR ORAL USE ONLY. SHAKE WELL BEFORE USING.