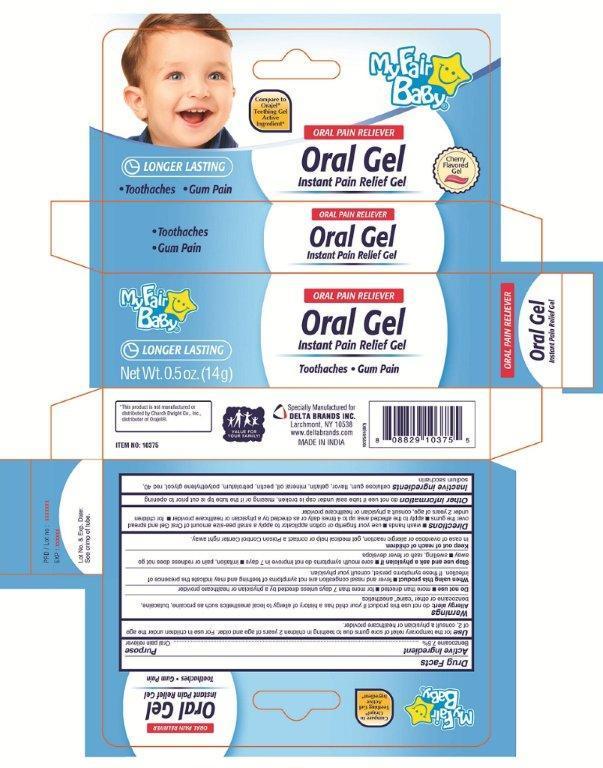
Uses
for the temporary relief of sore gums due to teething in children 2 years of age and older. For use in children under the age of 2, consult a physician or healthcare provider.
Warnings
Allergy alert: do not use this product if your baby has a history of allergy to local anesthetics such as procaine, butacaine or other "caine" anesthetics
Do not use
■ more than directed ■ for more than 7 days unless directed by a physician or healthcare provider
When using this product
■ fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms persist, consult your physician
Stop use and ask a physician if
sore mouth symptoms do not get better in 7 days ■ irritation, pain or redness does not go away ■ swelling, rash or fever develops
Keep out of reach of children
In case of overdose or allergic reaction get medical help or contact a Poison Control Center right away
Directions
■ wash your hands ■ use your fingertip or cotton applicator to apply a small pea-size amount of Oral Gel and spread over gums ■ apply to affected area up to 4 times daily or as directed by a physician or healthcare provider ■ for children under 2 years of age, consult a physician or health care provider
Other information
do not use if tube seal under cap is broken, missing or if the tube tip is cut prior to opening