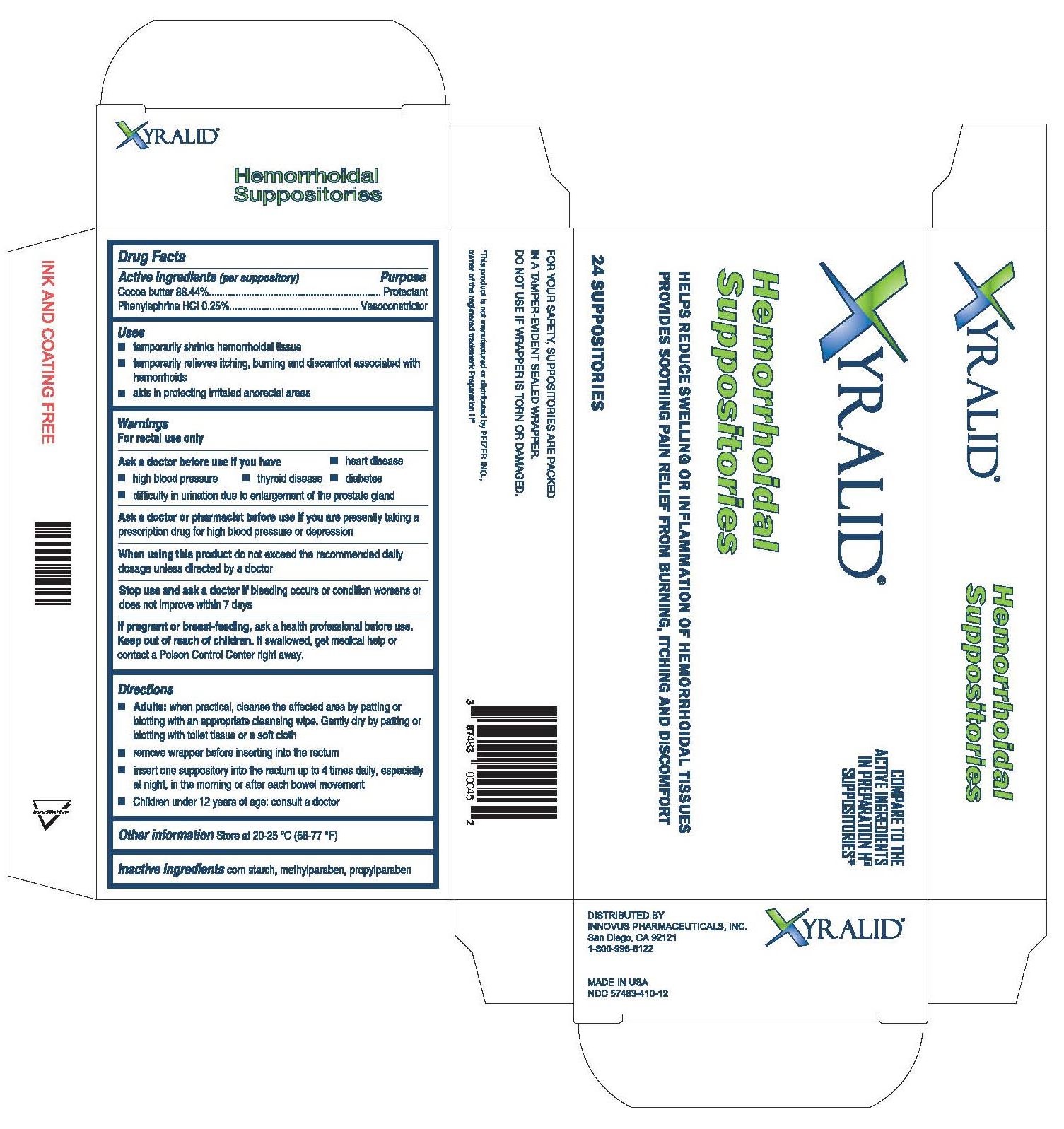
Active ingredients
Active Ingredients Purpose
Cocoa butter 88.50%...................................................................Protectant
Phenylephrine hydrochloride 0.26%..............................................Vasoconstrictor
Uses
- temporarily shrinks hemorrhoidal tissue
- temporarily relieves itching, burning, and discomfort associated with hemorrhoids
- aids in protecting irritated anorectal areas
Ask a doctor or pharmacist before use if you are
Ask a doctor or pharmacist before use if you are presently taking a prescription drug for high blood pressure or depression
Ask a doctor before use if you have
-heart disease -high blood pressure
-thyroid disease -diabetes
-difficulty in urination due to enlargement of the prostate gland
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-Adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a toilet tissue or a soft cloth.
-remove the wrapper before inserting into the rectum.
-insert one suppository into the rectum up to 4 times daily, especially at night, in the morning or after each bowel movement.
-Children under 12 years of age: consult a doctor