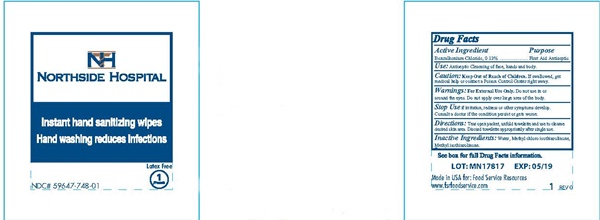
Caution: Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Warnings:
For external use only. Do not use in or around the eyes.Do not apply over large area of the body.
Stop Use
if irritation, redness or other symptoms develop. Consult a doctor if the condition persists or gets worse.
Directions:
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appopriately after single use.
NORTHSIDE HOSPITAL - Instant hand sanitizing wipes - product label
NH
NORTHSIDE HOSPITAL
Instant hands sanitizing wipes
Hand washing reduces Infections
Latex Free
1 Pouch
NDC# 59647-748-01
See box for full Drug Facts information
LOT: MN17817 EXP: 05/19
Made in the USA for: Food Service Resources
www.fsrfoodservice.com 1 REV 0