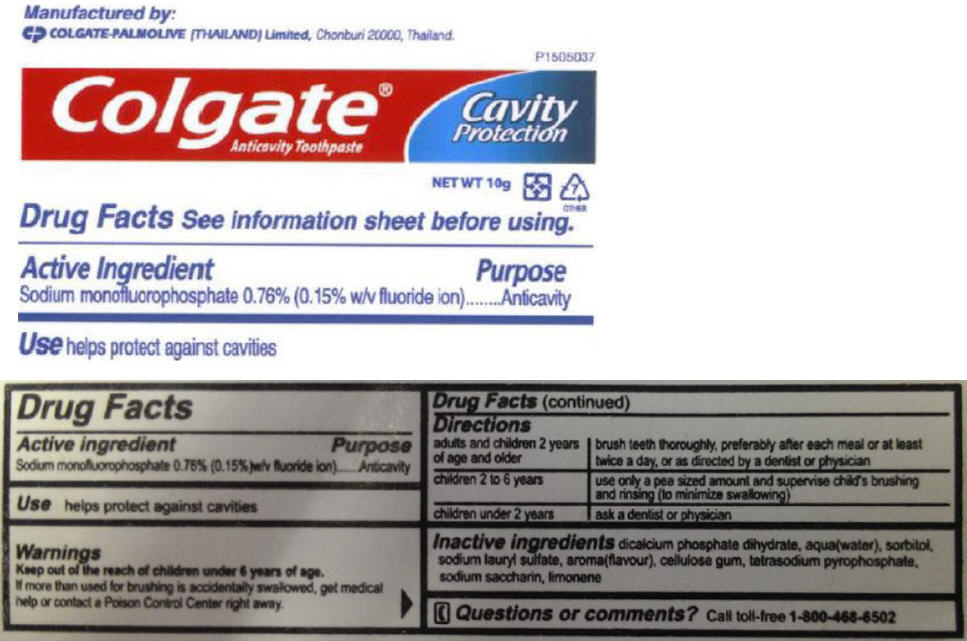
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
dicalcium phosphate dihydrate, aqua(water), sorbitol, sodium lauryl sulfate, aroma(flavour), cellulose gum, tetrasodium pyrophosphate, sodium saccharin, limonene
PRINCIPAL DISPLAY PANEL - 10 g Tube Label
Colgate®
Anticavity Toothpaste
Cavity
Protection
NET WT 10g
PRINCIPAL DISPLAY PANEL - Kit Label
GRACIE
SQUARE
HOSPITAL
Item #: GSHAMEN01
Description: Amenity Kit
PO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 20 KIT/CS
Carton #: X of XX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: NN x NN x NN CM
Assembled in China
Components Made in China:
- Shampoo/Conditioner
- Body Wash
- Body Lotion
- Alcohol-Free Mouthwash
- Hair Brush
- Toothbrush
- Ear Plugs
- Lip Balm
- Kit Case
Component Made in Thailand:
- Toothpaste
GRACIE
SQUARE
HOSPITAL
We are pleased to
present this welcome kit
for your comfort and
convenience during your
stay.
Thank you for choosing
Gracie Square Hospital
for your healthcare!
Contents/Origins:
2.0 fl. oz. Shampoo/Conditioner
2.0 fl. oz. Body Wash
2.0 fl. oz. Body Lotion
2.2 fl. oz. Alcohol-Free Mouthwash
0.15 oz. net wt. Lip Balm
Hair Brush, Toothbrush, Ear Plugs,
and Kit Case: Made in China
Toothpaste: Made in Thailand
Lot #:
Exp:
Distributed by:
ASP Global, LLC
3450 Atlanta Industrial Parkway
Atlanta, GA 30331
GSHAMEN01 Rev 2
GRACIE
SQUARE
HOSPITAL
