DESCRIPTION
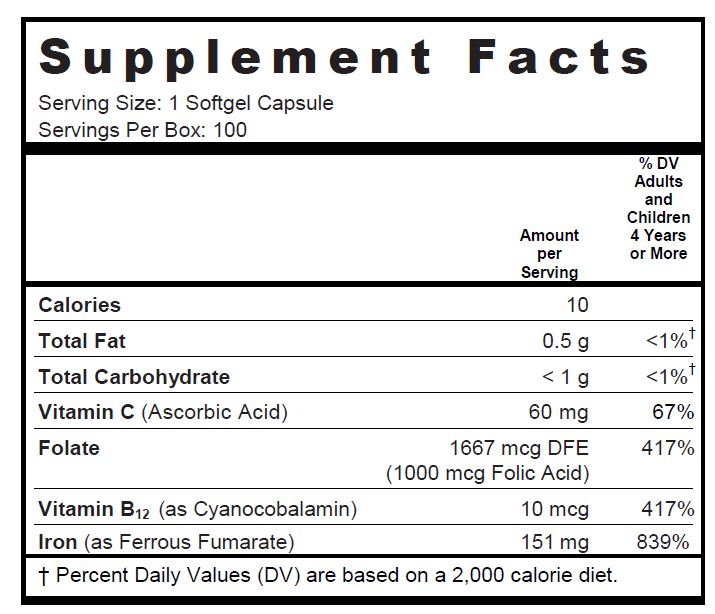
Other Ingredients: Soybean Oil, Gelatin, Glycerin, Purified Water, Yellow Beeswax, Soy Lecithin, Ethyl Vanillin, Titanium Dioxide, FD&C Red #40, FD&C Yellow #5, FD&C Blue #1
THIS PRODUCT CONTAINS SOY.
Trigels-F Forte is a prescription vitamin used for the dietary management of patients with nutritional deficiencies or are in need of nutritional supplementation.
CONTRAINDICATIONS
Trigels-F Forte should not be used by patients with a known hypersensitivity to any of the listed ingredients including soy. All iron compounds are contraindicated in patients with hemochromatosis, hemosiderosis, or hemolytic anemias.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
Do not exceed recommended dose. The type of anemia and the underlying cause or causes should be determined before starting therapy with Trigels-F Forte. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic Acid: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible patients. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid, as well as possibly the use of other forms of folates, including reduced folates. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with cyanocobalamin.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhea, tarry stools melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
DESCRIPTION
Trigels-F Forte is a brown, oblong shaped softgel with laser imprinted “TL518” containing dark brown paste.
HOW SUPPLIED
Trigels-F Forte is available in child resistant blister packs of 100 (10 x 10) softgel capsules.
Product Code: 13811-518-10
STORAGE
Store at 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Protect from moisture and excessive heat.
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-TRIGELSF-00001-1 Rev. 11/2021
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005
www.trigenlab.com

