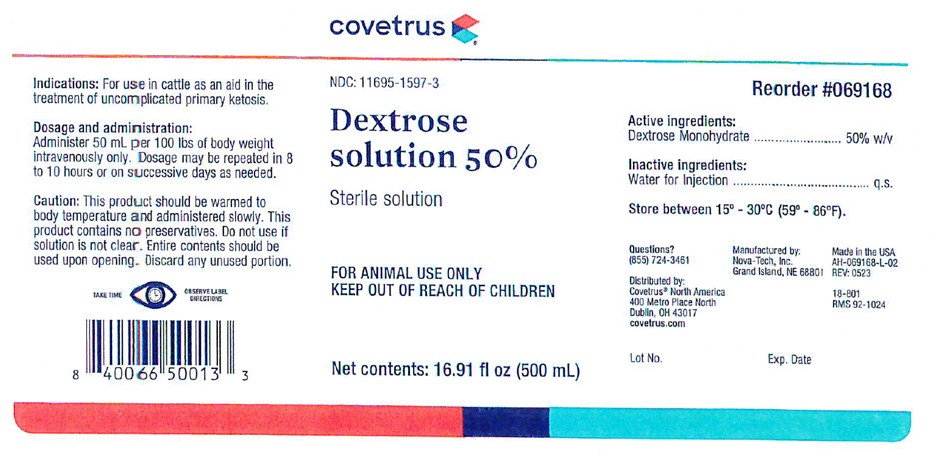
Dosage And Administration:
Administer 50 mL per 100 lbs of body weight intravenously only. Dosage may be repeated in 8 to 10 hours or on successive days as needed.
Caution:
This product should be warmed to body temperature and administered slowly. This product contains no preservative. Do not use if solution is not clear. Entire contents should be used upon opening. Discard any unused portion.
NDC: 11695-1597-3
18-801
RMS 92-1024
Volume: 16.907 (500 mL)
Questions? (855) 724-3461
Reorder #069168
Manufactured by:
Nova-Tech, Inc.
Grand Island, NE 68801
Distributed by:
Covetrus North America
400 Metro Place North
Dublin, OH 43017
covetrus.com
Made in the USA
AH-069168-L-02
Rev: 0523
Lot No. Exp. Date