Warnings
For external use only
When using this product
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration
Directions
See package insert for full prescribing information
Prime can before initial use: See package insert Before Each Use: Shake vigorously
During Use: Holding can upright, dispense into palm of hand and apply to affected area as directed by physician.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
Other Information
- Store at room temperature 15°-25° C (59°-77° F)
- Protect from freezing
- Store upright
Inactive Ingredients
BHT, C12-15 alkyl benzoate, cetostearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, povidone, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10, stearic acid, trolamine. Also contains: Propellant HFA-134A (1, 1, 1, 2-tetrafluoroethane).
Questions? 866-696-8525
Manufactured for:
PruGen, Inc.
Pharmaceuticals
18899 North Thompson Peak Parkway
Scottsdale, Arizona 85255 REV 1.2
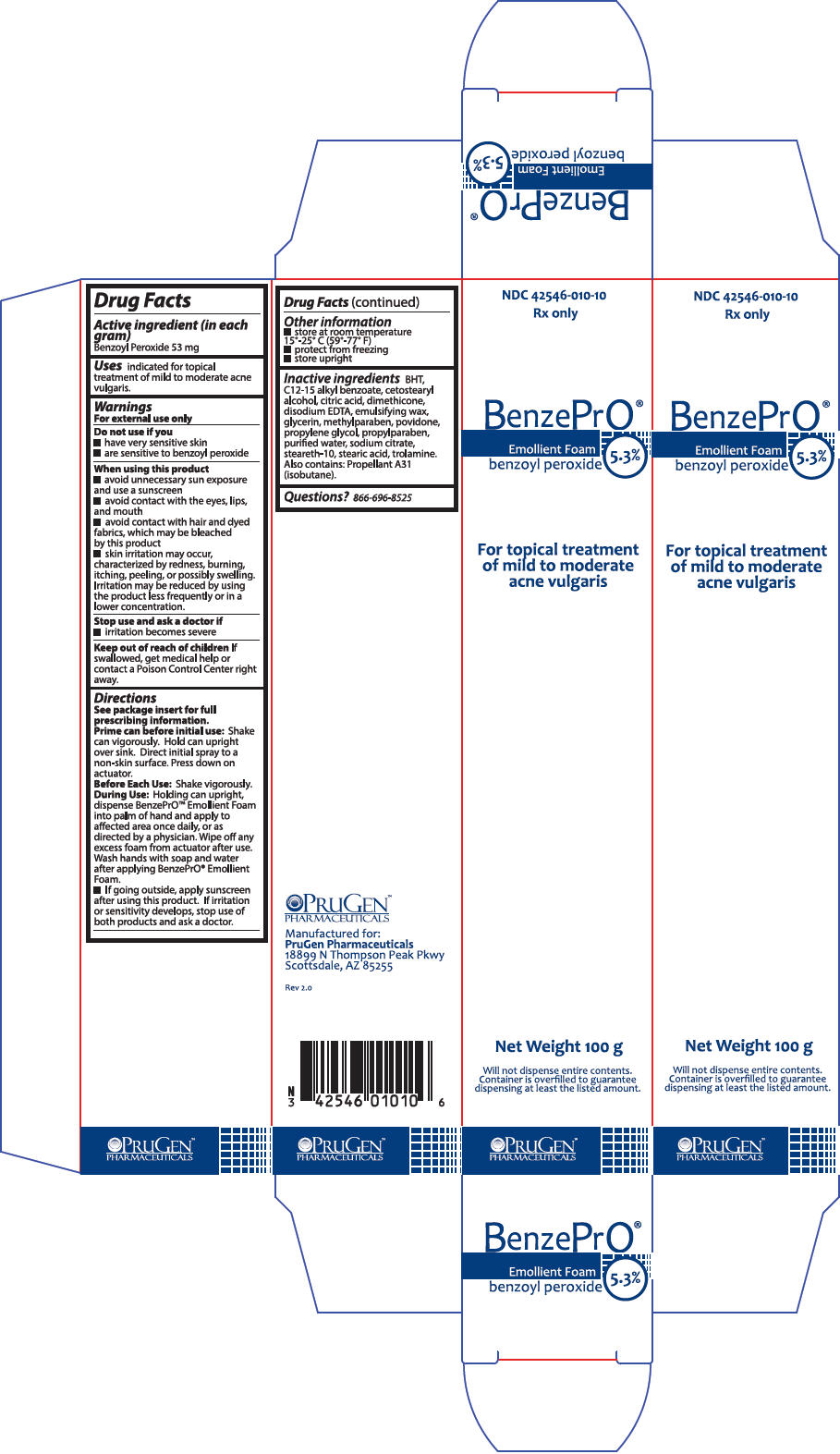
PRINCIPAL DISPLAY PANEL - 100 g Can Box
NDC 42546-010-10
Rx only
BenzePrO®
Emollient Foam
benzoyl peroxide
5.3%
For topical treatment
of mild to moderate
acne vulgaris
Net Weight 100 g
Will not dispense entire contents.
Container is overfilled to guarantee
dispensing at least the listed amount.
PRUGEN™
PHARMACEUTICALS