Water
Dipropylene Glycol
Agar
Sodium Polyacrylate
Cellulose Gum
Polyacrylic Acid
Tartaric acid
Phenoxyethanol
Disodium EDTA
Aluminum Glycinate
Adenosine
Cetyl Ethylhexanoate
Hydrogenated Polyisobutene
Caffeine
Fragrance
Aloe Barbadensis Leaf Extract
Butylene glycol
PHOSPHATIDYLCHOLINE
Xanthan Gum
Hydrolyzed Collagen
Coco-Glucoside
Alcohol
Chenopodium Quinoa Seed Extract
Glaucine
Caprylyl Glycol
Anemarrhena Asphodeloides Root Extract
Sodium Hyaluronate
TOCOPHEROL
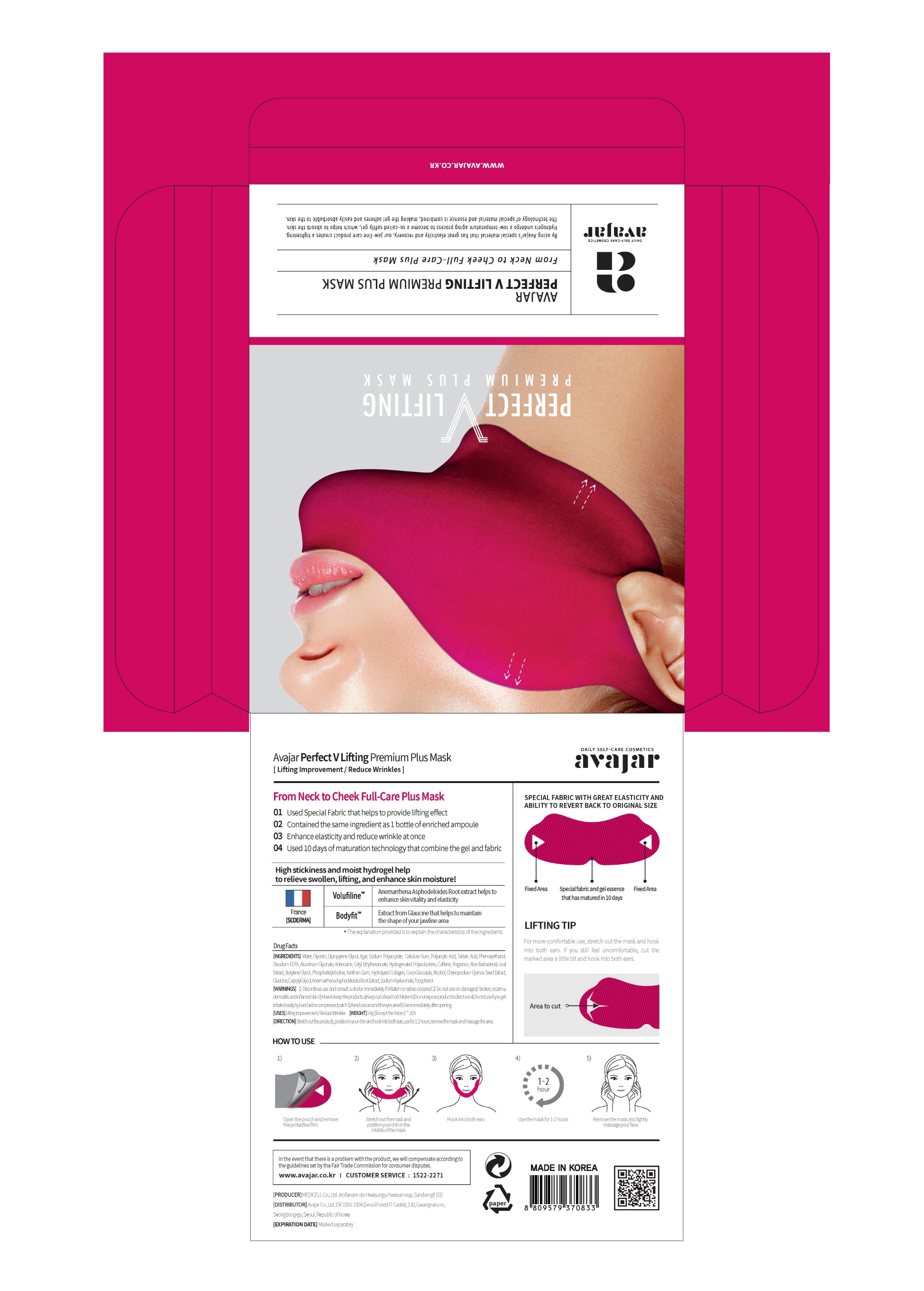
1. Open pouch and remove the film.
2. Hold each ends of the mask with hands, STRETCH and place the middle on the chin.
3. After matching your chin to the middle of the mask, hook onto the ears.
4. After use, remove mask and lighly massage both cheeks.
1. If the following symptoms occur after product use, stop using the product immediately and consult a dermatologist (continuous use can exacerbate the symptoms).
1) Occurrence of red spots, swelling, itchiness, and other skin irritation
2) If the symptoms above occur after the application area is exposed to direct sunlight
2. Do not use on open wounds, eczema, and other skin irritations
3. Precaution for Storage and Handling
1) Close the lid after use
2) Keep out of reach of infants and children
3) Do not to store in a place with high/low temperature and exposed to direct sunlight