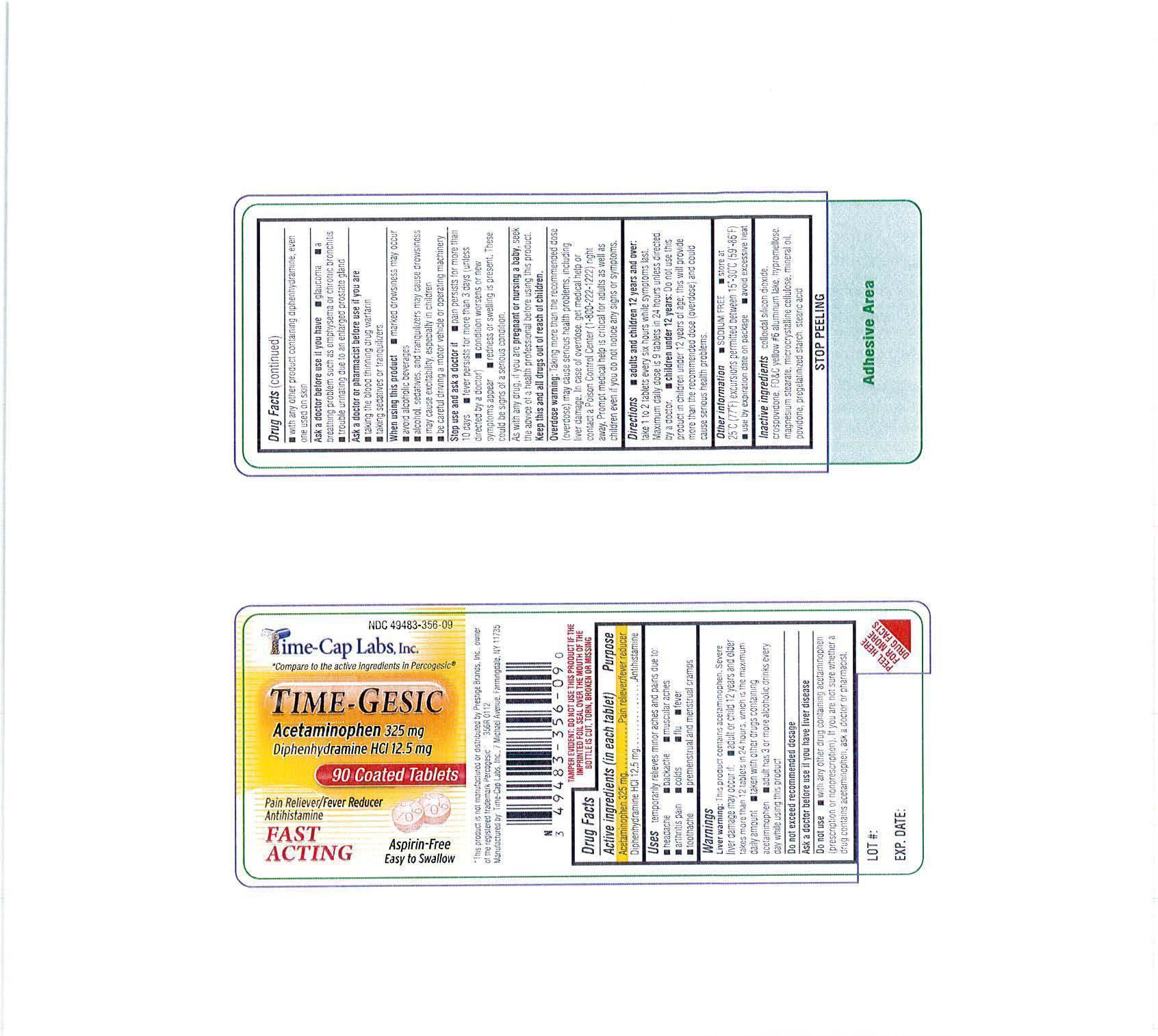
Do not use
- WITH ANY OTHER DRUG CONTAINING ACETAMINOPHEN(PRESCRIPTION OR NONPRESCRIPTION). IF YOU ARE NOT SURE WHETHER A DRUG CONTAINS ACETAMINOPHEN, ASK A DOCTOR OR PHARMACIST.
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
Stop use and ask a doctor if
pain persists for more than 10 days; fever persists for more than 3 days (unless directed by a doctor); condition worsens or new symptoms appear; redness or swelling is present. These could be signs of a serious condition.
If pregnant or nursing, as with any drug, seek the advise of a health professional before using this product.
Directions
adults and children 12 years and over: take 1 to 2 tablets every six hours while symptoms last. Maximum daily dose is 9 tablets in 24 hours unless directed by a doctor.
children under 12 years: Do not use this product in children under 12 years of age; this will provide more than the recommended dose (overdose) and could cause serious health problems.
Other information
- store at 25° C (77° F) excursions permitted between 15°-30° C (59°-86° F)
- use by expiration date on package
- avoid excessive heat
- sodium free
colloidal silicon dioxide, crospovidone, fd&C yellow #6 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, povidone, pregelatinized starch, stearic acid
Uses: temporarily releives mnor aches and pains due to:
headache, backache, muscular aches, arthritis pain, colds, flu, fever, toothache, premenstrual cramps.
Warnings:
Liver warning: This product contains acetaminophen. Severe liver damage may occur if: adult or child 12 years and older takes more than 12 tablets in 24 hours, which is the maximum daily amount; taking with other durgs containing acetaminophen; adult has 3 or more alcoholic drinks every day while using this product.