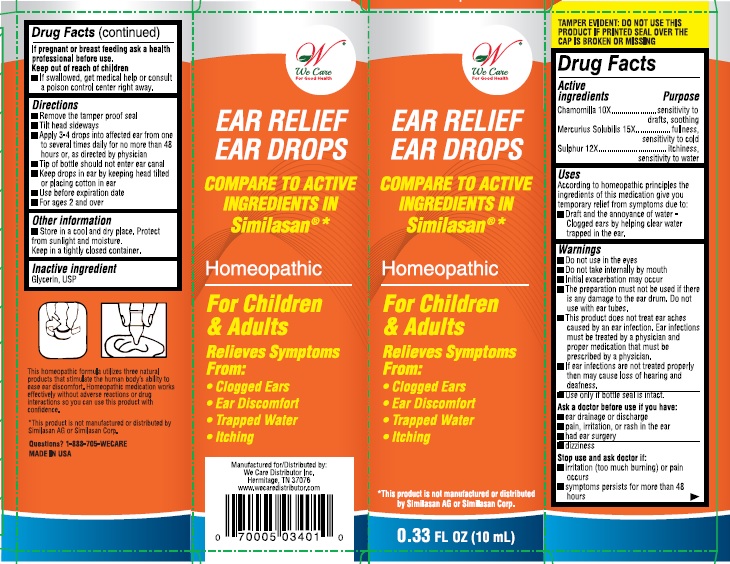
Uses:
According to homeopathic principles the ingredients of this medication give you temporary relief from symptoms due to:
- drafts and the annoyance of water-clogged ears by helping clear water trapped in the ear.
Warnings:
- Do not use in the eyes.
- Do not take internally by mouth
- Initial exacerbation may occur.
- The preparation must not be used if there is any damage to the ear drum, do not use with ear tubes.
- This product does not treat ear aches caused by an ear infection. Ear infections must be treated by a physician and proper medication that must be prescribed by a physician.
- If ear infections are not treated properly then may cause loss of hearing and deafness.
- Use only if bottle seal in intact.
Ask a doctor before use if you have:
- ear drainage or discharge
- pain, irritation, or rash in the ear
- had ear surgery
- dizzines
Stop use and ask a doctor if:
- irritation (too much burning) or pain occurs
- symptoms persist for more than 48 hours
Keep out of reach of children.
If swallowed, get medical help or consult a Poison Control Center right away.
Directions:
- Remove tamper proof seal
- Tilt head sideways
- Apply 3-4 drops into affected ear from one to several times daily for no more than 48 hours, or as directed by a physician
- Tip of the bottle should not enter ear canal
- Keep drops in ear by keeping head tilted or placing cotton in ear.
- Use before expiration date
- For ages 2 and over