Warnings
For external use only
Other information
- for additional information, see Safety Data Sheet (SDS)
- for emergency medical information in USA and Canada, call 1 800 328 0026
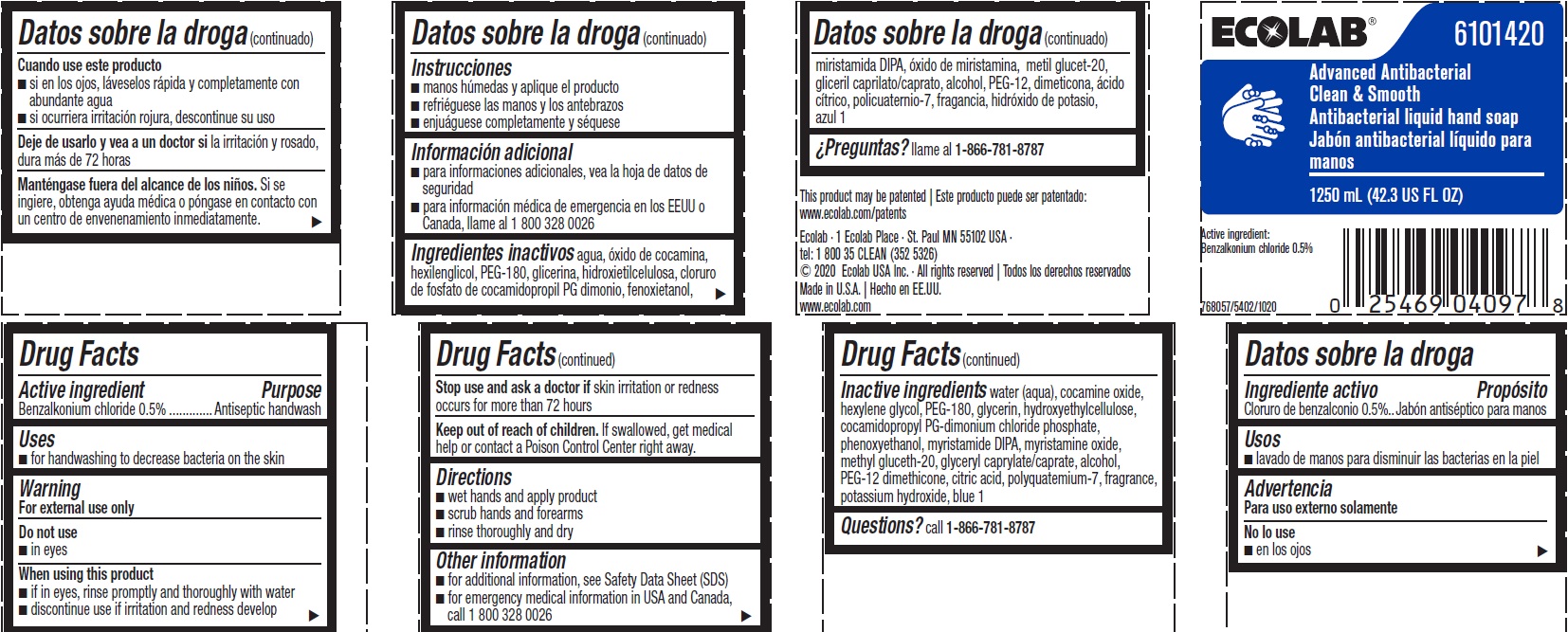
Inactive ingredients water (aqua), cocamine oxide, hexylene glycol, PEG-180, glycerin, hydroxyethylcellulose, cocamidopropyl PG-dimonium chloride phosphate, phenoxyethanol, myristamide DIPA, myristamine oxide, methyl gluceth-20, glyceryl caprylate/caprate, alcohol, PEG-12 dimethicone, citric acid, polyquatemium-7, fragrance, potassium hydroxide, blue 1
Representative label & Primary display panel
ECOLAB 6101420
Advanced Antibacterial
Clean & Smooth
Antibacterial liquid hand soap
1250 mL (42.3 US FL OZ)
Active Ingredient
Benzalkonium chloride 0.5%
This product may be patented | Este producto puede ser patentado: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA ·
tel: 1 800 35 CLEAN (352 5326)
© 2020 Ecolab USA Inc. · All rights reserved | Todos los derechos reservados
Made in U.S.A. | Hecho en EE.UU.
www.ecolab.com