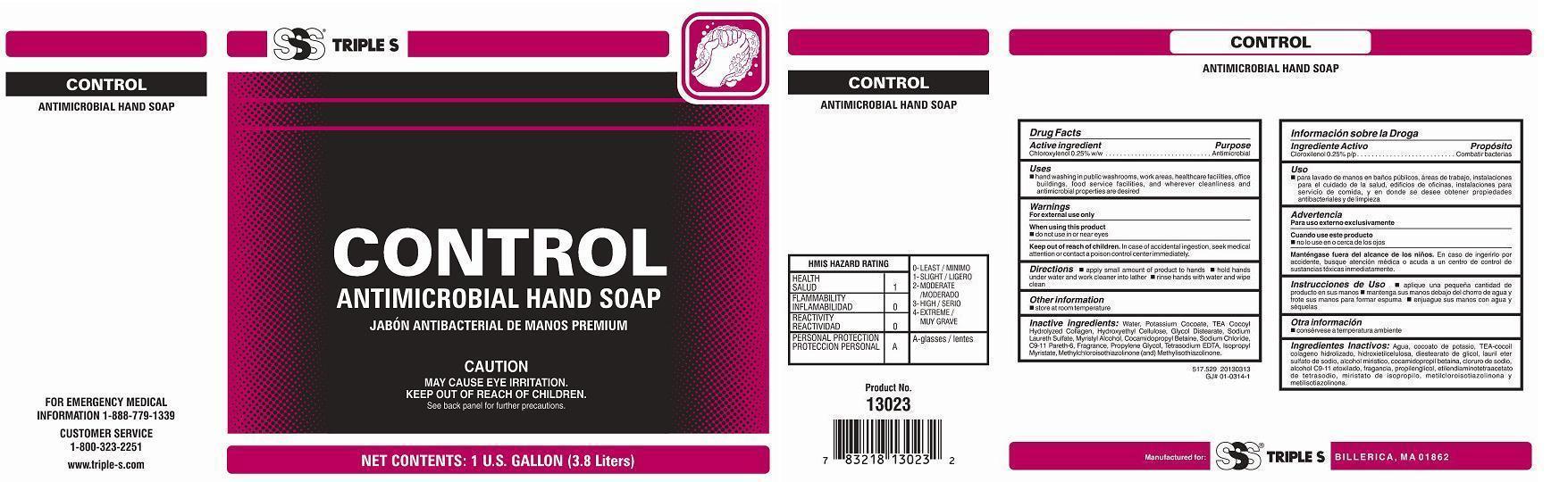
Keep out of reach of children
In case of accidental ingestion, seek medical attention or contact a poison control center immediately.
Uses
Hand washing in public washrooms, work areas, healthcare facilities, office buildings, food
service facilities, and wherever cleanliness and antimicrobial properties are desired
Directions
Apply small amount of product to hands
Hold hands under water and work cleaner into lather
Rinse hands with water and wipe clean
Inactive ingredients:
Water, Potassium Cocoate, TEA-Cocoyl Hydrolyzed Collagen, Hydroxyethyl Cellulose, Glycol Distearate, Sodium Laureth Sulfate, Myristyl Alcohol, Cocoamidopropyl Betaine, Sodium Chloride, C9-11 Pareth-6, Fragrance, Propylene Glycol, Tetrasodium EDTA, Isopropyl Myristate, Methylchloroisothiazolinone (and) Methylisothiazolinone.
PRINCIPAL DISPLAY PANEL - 3,785 mL Bottle Label
SSS® triple S
CONTROL
Antimicrobial Hand Soap
Jabón Antibacterial de Manos Premium
CAUTION: MAY CAUSE EYE IRRITATION. KEEP OUT OF REACH OF CHILDREN. See back panel for further precautions
Net Contents: 1 U.S. gallon / 3.78 liters
Manufactured for: SSS® triple S Billerica, MA 01862