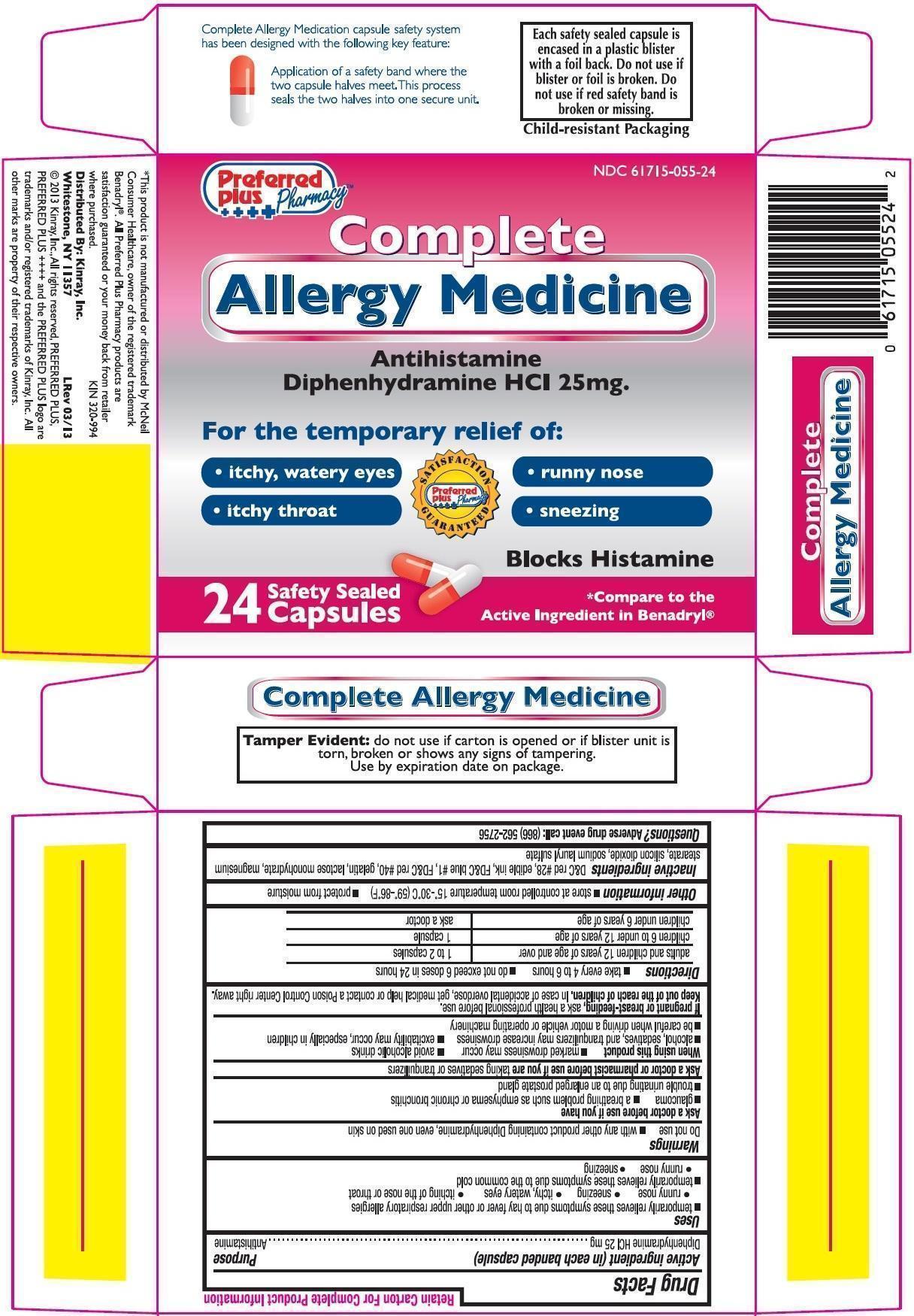
Uses
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- runny nose
- sneezing
Warnings
Ask a doctor before use if you have
- Glaucoma
- A breathing problem such as emphysema or chronic bronchitis
- Trouble urinating due to an enlarged prostate gland
Directions
- take every 4 to 6 hours
- do not exceed 6 doses in 24 hours
| Adults and children 12 years of age and over |
1 to 2 capsules |
|
Children 6 to under 12 years of age |
1 capsule |
| Children under 6 years of age | ask a doctor |
Other information
- Store at controlled room temperature 150-300C (590-860F)
- Protect from excessive moisture
Inactive ingredients
D & C Red #28, FD & C Blue #1, FD & C Red #40, Gelatin, Lactose Monohydrate, Magnesium Stearate, Silicon Dioxide, Sodium Lauryl Sulfate
Principal Display Panel
Complete Allergy Medicine
Antihistamine
Diphenhydramine HCl 25mg
For the temporary relief of:
- itchy, watery eyes
- runny nose,
- itchy throat
- sneezing
Blocks Histamine
*Compare to the Active Ingredient in Benadryl®.
Safety Sealed Capsules
*This product is not manufactured or distributed by McNeail Consumer Healthcare, owner of the registered trademark Benadryl®. All Preferred Plus Pharmacy are satisfaction guaranteed or your money back from retailer where purchased.
Distributed By: Kinray, Inc.
Whitestone, NY 11357
©2013 Kinray, Inc., All rights reserved, PREFERRED PLUS, PREFERRED PLUS ++++ and the PREFERRED PLUS logo are trademarks of Kinray, Inc. All other marks are property of their respective owners.
Product Label