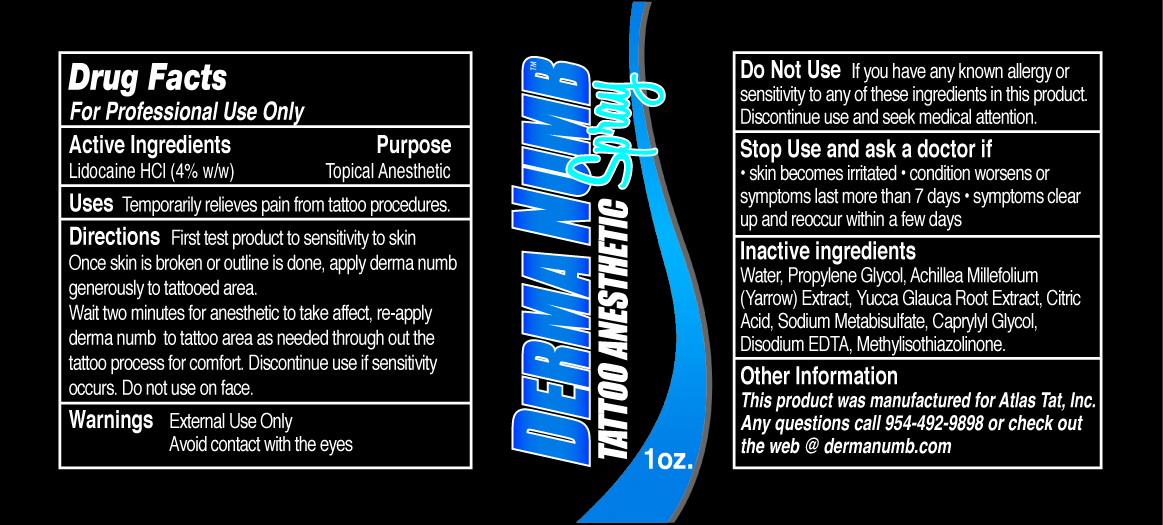
Directions
First test product to sensitivity to skin.
Once skin is broken or outline is done, apply derma numb generously to tattooed area.
Wait two minutes for anesthetic to take affect, re-apply derma numb to tattoo area as needed through out the tattoo process for comfort. Discontinue use if sensitivity occurs. Do not use on face.
Do Not Use
If you have any known allergy or sensitivity to any of these ingredients in this product. Discontinue use and seek medical attention.
Stop Use and ask a doctor if
• skin becomes irritated • condition worsens or symptoms last more than7 days • symptoms clear up and reoccur with a few days
Inactive ingredients
Water, Propylene Glycol, Achillea Millefolium (Yarrow) Extract, Yucca Glauca Root Extract, Citric Acid, Sodium Metabisulfate, Caprylyl Glycol, Disodium EDTA, Methylisothiazolinone.