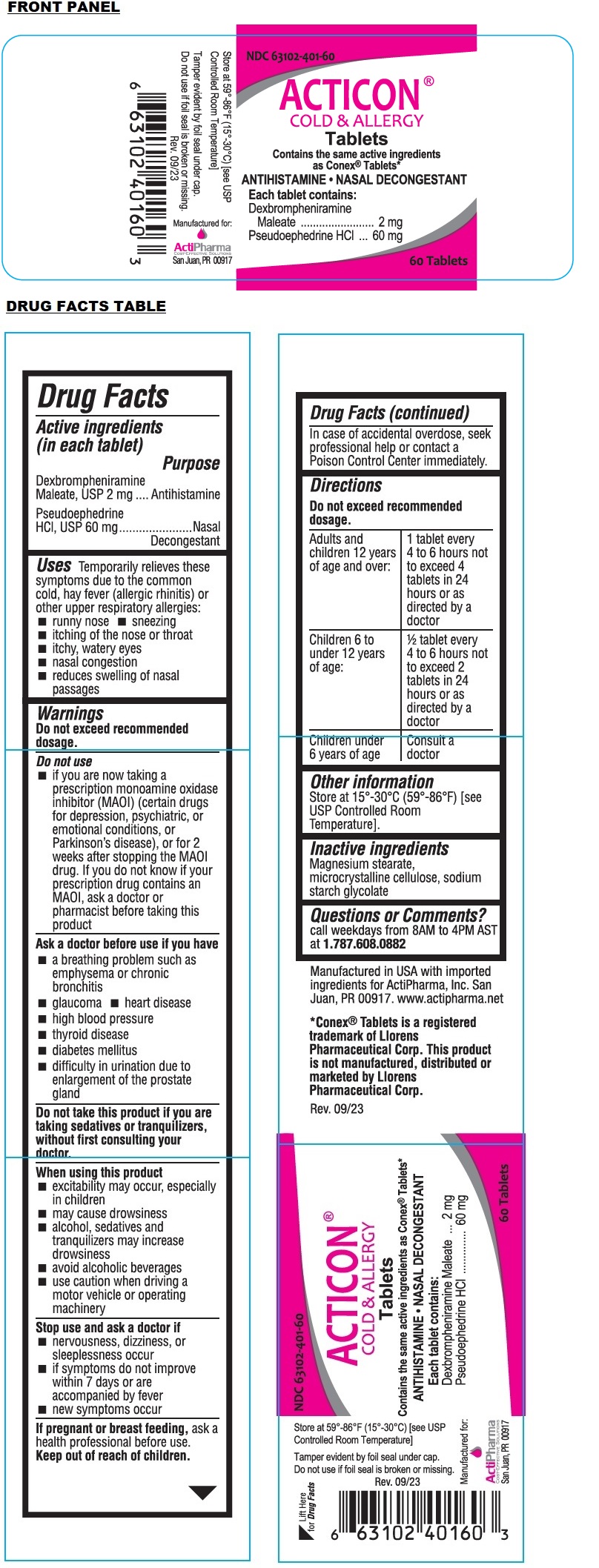
Active ingredients (in each tablet)
Dexbrompheniramine Maleate, USP 2 mg
Pseudoephedrine HCl, USP 60 mg
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
• runny nose • sneezing
• itching of the nose or throat
• itchy, watery eyes
• nasal congestion
• reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
Ask a doctor before use if you have
• a breathing problem such as emphysema or chronic bronchitis
• glaucoma • heart disease
• high blood pressure
• thyroid disease
• diabetes mellitus
• difficulty in urination due to enlargement of the prostate gland
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
• excitability may occur, especially in children
• may cause drowsiness
• alcohol, sedatives and tranquilizers may increase drowsiness
• avoid alcoholic beverages
• use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
• nervousness, dizziness, or sleeplessness occur
• if symptoms do not improve within 7 days or are accompanied by fever
• new symptoms occur
If pregnant or breast feeding, ask a health professional before use.
Directions
Do not exceed recommended dosage.
| Adults and children 12 years of age and over: | 1 tablet every 4 to 6 hours not to exceed 4 tablets in 24 hours or as directed by a doctor |
| Children 6 to under 12 years of age: | 1/2 tablet every 4 to 6 hours not to exceed 2 tablets in 24 hours or as directed by a doctor |
| Children under 6 years of age | Consult a doctor |
Contains the same active ingredients as Conex® Tablets*
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Manufactured in USA with imported ingredients for ActiPharma, Inc. San Juan, PR 00917. www.actipharma.net
*Conex® Tablets is a registered trademark of Llorens Pharmaceutical Corp. This product is not manufactured, distributed or marketed by Llorens Pharmaceutical Corp.