Active Ingredient
Lidocaine HCl 0.5%
Purpose
Topical analgesic
Active Ingredient
Benzalkonium chloride 0.13%
Purpose
Topical antiseptic
Uses
For the temporary relief of pain and itching associated with sunburn, minor burns, insect bites, minor skin irritation, cuts, scrapes
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body or on deep puncture wounds, animal bites, or serious burns
- in large quantities, particularly over raw surfaces or blistered areas
Stop use and ask a doctor if
- the condition gets worse
- condition clears up and recurs within a few days
- condition persists for more than 7 days
If pregnant or breast feeding,
ask a health care professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 2 years and over:
- clean the affected area
- apply a small amount of this product on the area 3 to 4 times daily
- may be covered with a sterile bandage
Children under 2 years:
Other Information
- store in a cool, dry area 15°-25° C (59°-79° F)
- tamper evident sealed packets
- do not use any opened or torn packets
Inactive Ingredients
Butylated Hydroxytoluene, Cetomacrogol, Cetostearyl Alcohol, Dimethicone, Glycerine, Glyceryl Monostearate, Isopropyl Myristate, Methylcellulose, Purified Water, Sodium EDTA, Sodium Methylparaben, Sodium Propylparaben
1165 Label

1165UB-10 Label
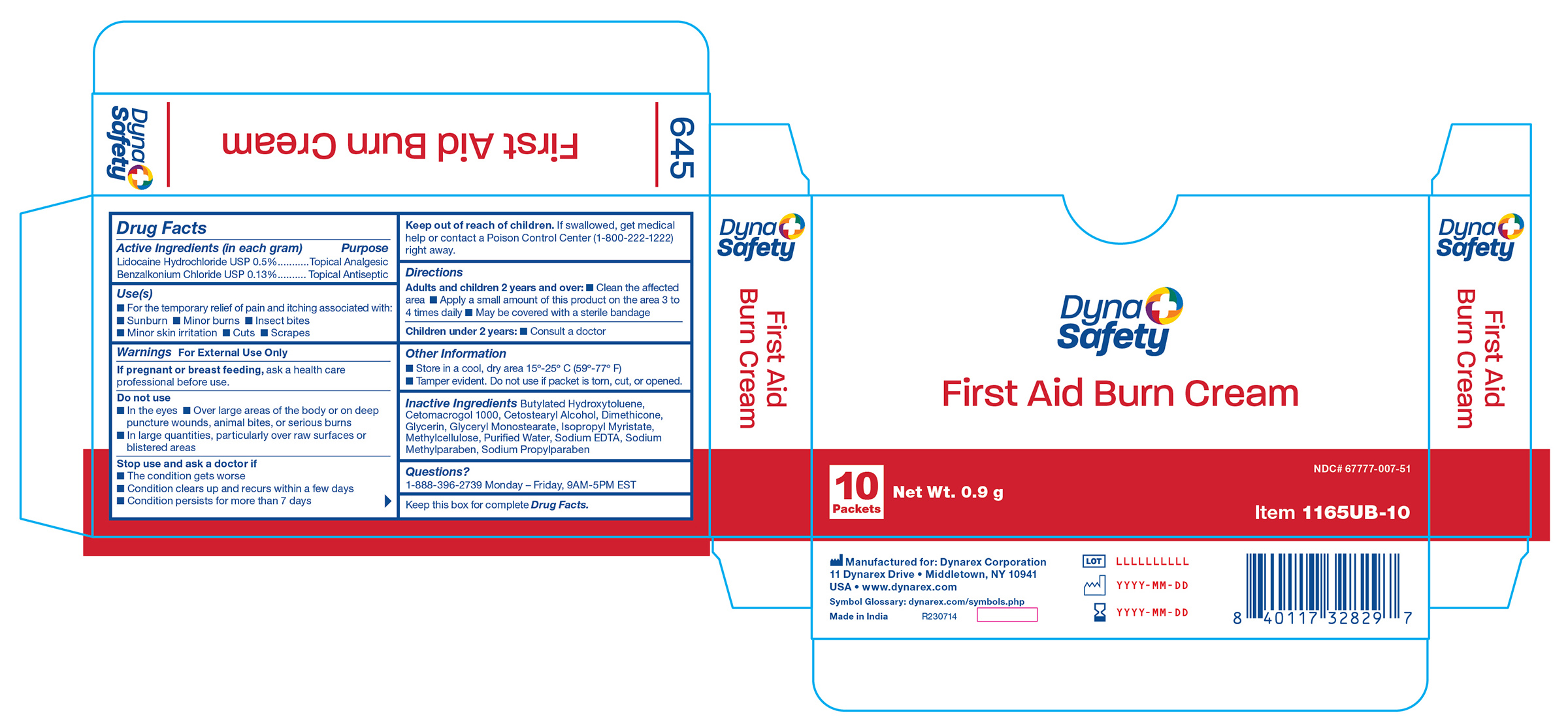 1165UB-10
1165UB-10
1165UB-25 Label
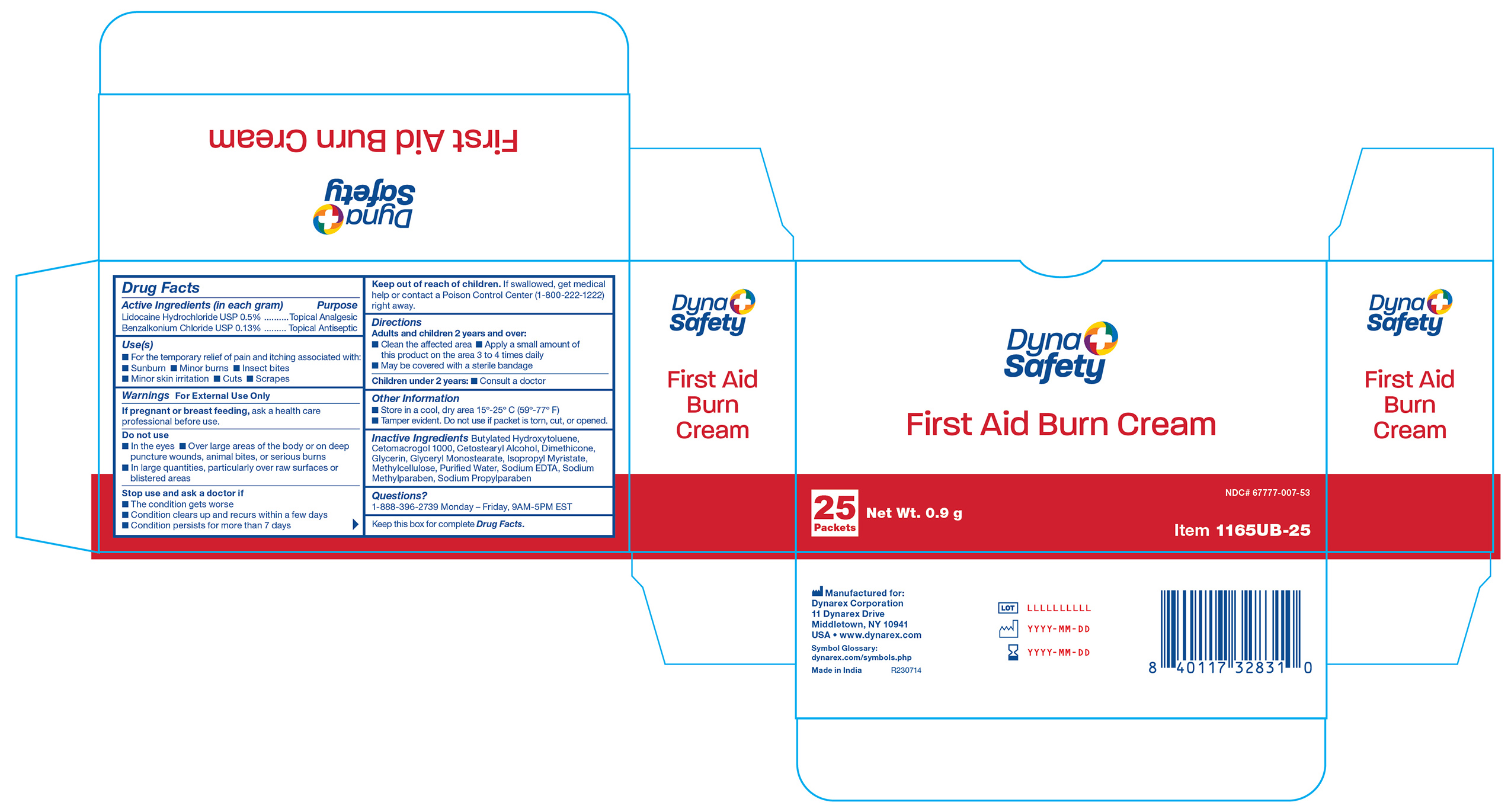

 1165UB-10
1165UB-10
