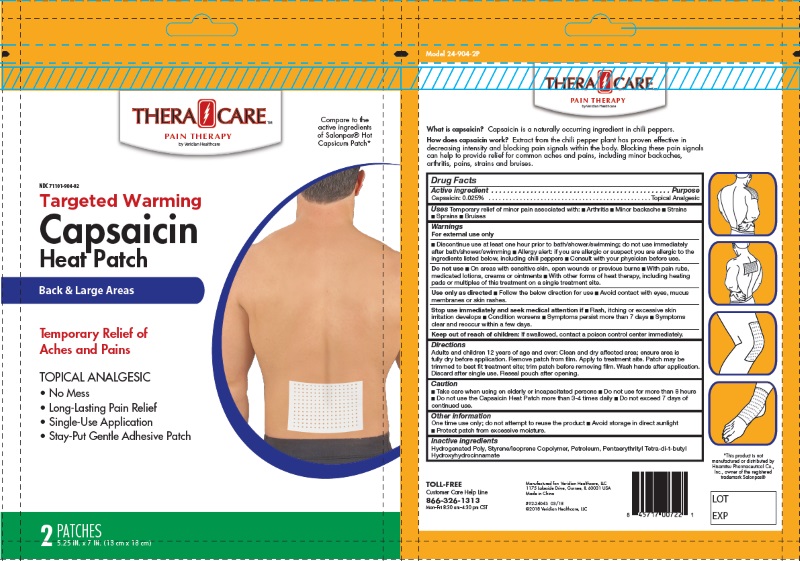
For External Use Only
- Discontinue use at least one hour prior to bath / shower / swimming; do not use immediatelty after bath / shower / swimming.
- Allergy alert: if you are allergic or suspect you are allergic to the ingredients listed below, including chili perppers.. consults with your physician before use.
Uses
Temporary relief of minor pain associated with:
- Arthiritis
- Minor backache
- Strains
- Sprains
- Bruises
Inactive Ingredients:
Hydrogenated Poly, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Petroleum, Styrene / Isoprene Copolymer
Directions for Use:
For Use on Adults and Childrens 12 Years of Age or Older
- Clean and dru affected area; ensure area is fully dry before application
- Remove patch from film
- Apply to treatement site
- Patch may be trimmerd to best fit treatment site; trim patch before removing film
- Wash hands after application
- Discard after signle use
- Take care when using on elderly ot incapacited persons
- Do not use more than 8 hours
- Do not use the Capsaicin Heat Patch more than 3-4 times daily
- Do not exceed 7 days of continue use
Storage and Care:
- One time use only; do not attempt to reuse the product
- avoid storage in direct sunlight
- Protect patch from excessive moisture
Do Not Use:
- On areas with sensitive skin, open wounds or previous burns
- with pain rubs, medicated lotions, creams or oinments
- with other forms of heat theraphy, including heating pads, or multiples of this treatment on a single treatement site