Warnings
Stop use and ask a health care practitioner if symptoms persist for more than five days or worsen. If pregnant or breastfeeding, ask a health care practitioner before use.
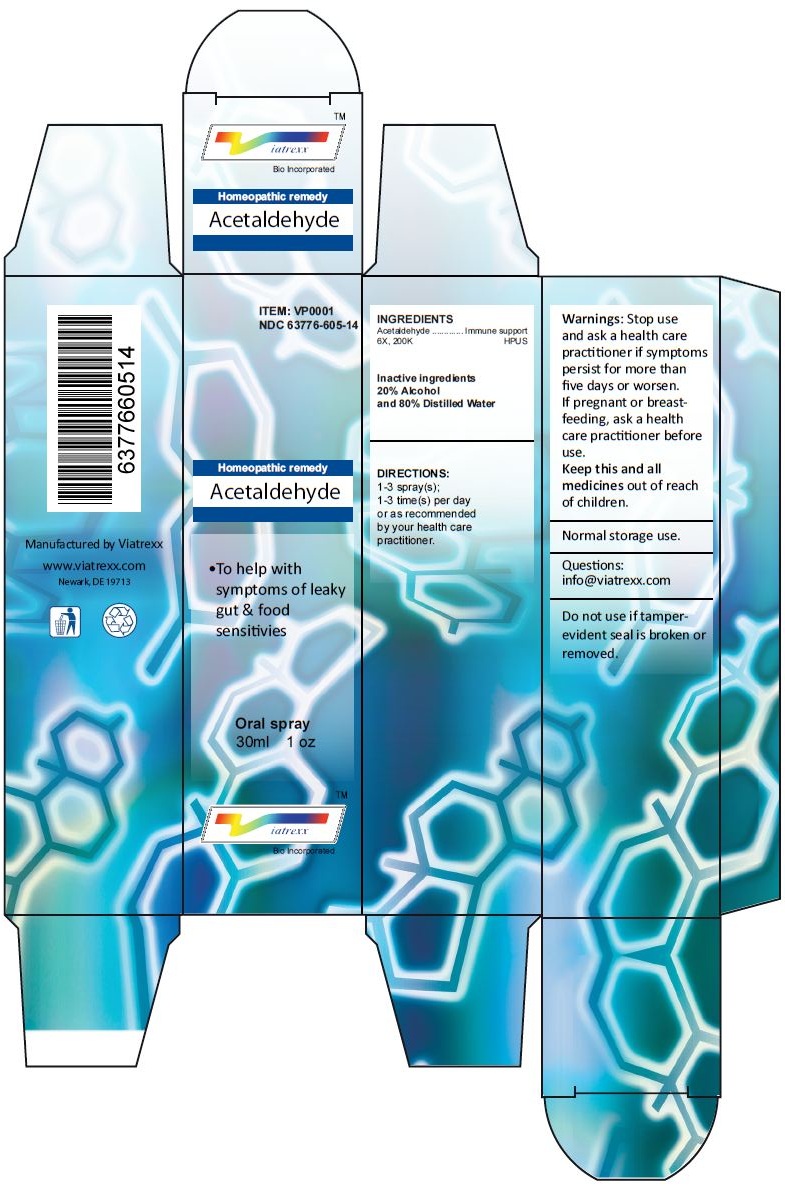
Principal Display Panel
ITEM: VP0001
NDC 63776-605-14
Homeopathic remedy
Acetaldehyde
• To help with symptoms of leaky gut & food sensitivies
Oral spray
30ml 1 oz
Viatrexx™ Bio Incorporated
Manufactured by Viatrexx
www.viatrexx.com
Newark, DE 19713
Acetaldehyde
30 ml
1 oz
Viatrexx™ Bio Incorporated
ITEM: VP0001
NDC: 63776-605-14
INDICATIONS:
To help with symptoms of leaky gut & food sensitivies
DIRECTIONS:
1-3 spray(s); 1-3 time(s) per day or as recommended by your health care practitioner.
Mfg. for
Viatrexx Bio Incorporated.
www.viatrexx.com
Newark, DE 19713
