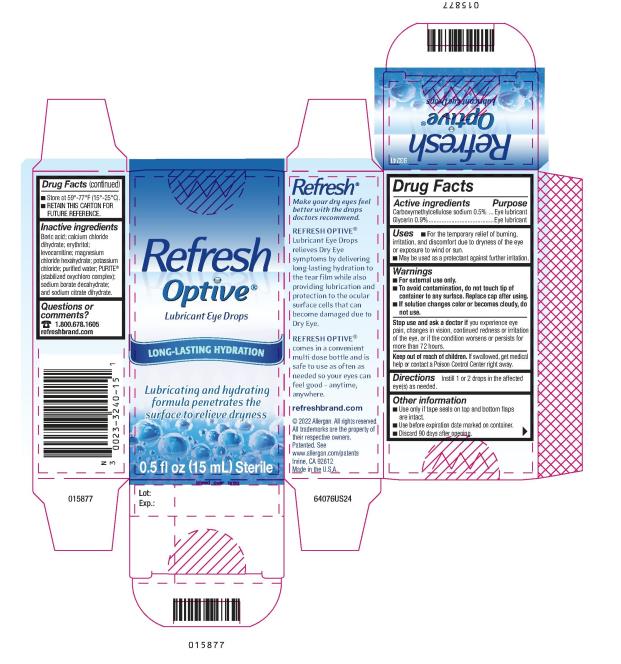
Active ingredients
Carboxymethylcellulose sodium 0.5%
Glycerin 0.9%
Purpose
Eye lubricant
Eye lubricant
Uses
- For the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
- May be used as a protectant against further irritation.
Warnings
-
For external use only.
-
To avoid contamination, do not touch tip of container to any surface. Replace cap after using.
-
If solution changes color or becomes cloudy, do not use.
Stop use and ask a doctor if
you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Instill 1 or 2 drops in the affected eye(s) as needed.
Other information
- Use only if tape seals on top and bottom flaps are intact.
- Use before expiration date marked on container.
- Discard 90 days after opening.
- Store at 59°-77°F (15°-25°C).
- RETAIN THIS CARTON FOR FUTURE REFERENCE.
Inactive ingredients
Boric acid; calcium chloride dihydrate; erythritol; levocarnitine; magnesium chloride hexahydrate; potassium chloride; purified water; PURITE® (stabilized oxychloro complex); sodium borate decahydrate; and sodium citrate dihydrate.
Questions or comments?
1.800.678.1605
refreshbrand.com
v1.0DFL3240
PRINCIPAL DISPLAY PANEL
0023-3240-15
Refresh
Optive®
Lubricant Eye Drops
LONG-LASTING HYDRATION
Lubricating and hydrating
formula penetrates the
surface to relieve dryness
0.5 fl oz (15 mL) Sterile
Allergan, Inc.
