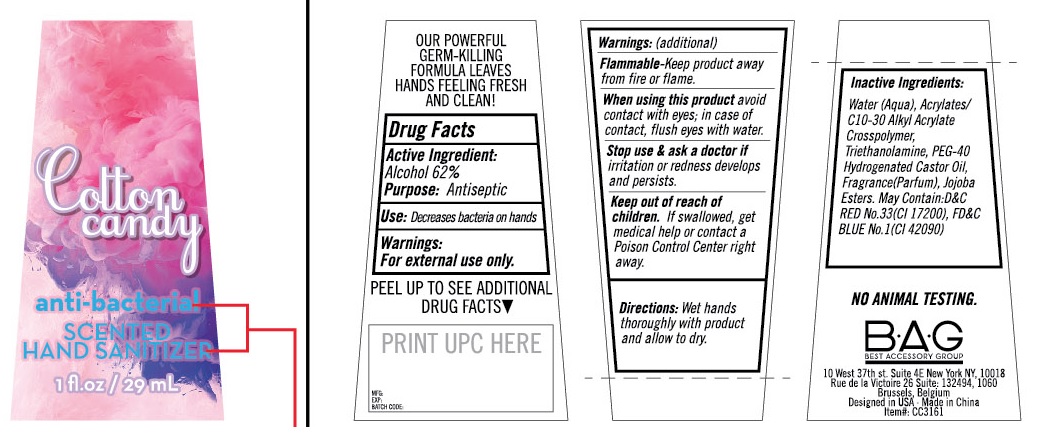
COTTON CANDY ANTI-BACTERIAL SCENTED HAND SANITIZER- alcohol solution
Best Accessory Group
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
OUR POWERFUL GERM-KILLING FORMULA LEAVES HANDS FEELING FRESH AND CLEAN !
Drug Facts
Active Ingredient:
Alcohol 62%
Use: Decreases bacteria on hands.
Warnings:
For External Use Only.
Flammable -Keep product away from fire or flame.
When using this product avoid contact with eyes; in case of contact, flush eyes with water.
Stop use & ask a doctor if irritation or redness develops and persists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions: Wet hands thoroughly with product and allow to dry.
Inactive Ingredients:
Water(Aqua), Acrylates/C10-30 Alkyl Acrylate Crosspolymer,Triethanolamine, PEG-40 Hydrogenated Castor Oil, Fragrance (Perfume), Jojoba Esters. May Contain: D&C RED No.33 (CI 17200), FD&C BLUE No. 1 (CI 42090)
NO ANIMAL TESTING
B•A•G
BEST ACCESSORY GROUP
10 West 37th st. Suite 4E New York NY, 10018
Rue de la Victoire 26 Suite: 132494, 1060
Brussels, Belgium
Designed in USA. Made in China
Item#: CC3161
PRINCIPAL DISPLAY PANEL - 29 mL Bottle Label
Cotton
candy
antibacterial
SCENTED
HAND SANITIZER
1 fl.oz - 29 mL