KIDS COLD AND MUCUS RELIEF EXPECTORANT- antimony pentasulfide and potassium iodide and polygala senega root syrup
Similasan Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
Antimonium sulphuratum aureum 12X
Purpose
loosens mucus, moist cough, chest congestion
Active Ingredient
Kali iodatum 12X
Purpose
cold, fever, sneezing, runny nose, head congestion
Active Ingredient
Senega offcinalis 8X
Purpose
expectorant, dry/hacking cough
Uses:
Temporarily relieves symptoms associated with the common cold, such as: mucus congestion, cough, fever, chest and head congestion.
Warnings:
- Initial exacerbation of symptoms may occur.
- Do not exceed recommended dosage.
- Not intended for children under 2.
- A persistent cough may be a sign of a serious condition.
Keep out of reach of children.
In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if:
- symptoms worsen or persist for more than 72 hours
Directions:
For children age 2 and over:
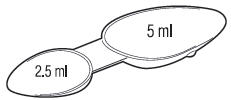
| AGE | DOSE | FREQUENCY |
| 2-12 | 2.5 ml | 3 to 6 times daily
(every 4 hours) |
| 12+ | 5 ml | 3 to 6 times daily
(every 4 hours) |
• Replace cap tightly after every use
Or use as directed by a licensed health care professional.
Other Information:
Active ingredients are manufactured according to homeopathic principles.
Inactive Ingredients:
Purified water, Sorbitol, Sodium chloride, Potassium sorbate, Citric acid
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com
www.SimilasanUSA.com
PRINCIPAL DISPLAY PANEL
NDC 59262-257-25
Similasan
Kids Cold & Mucus Relief
EXPECTORANT SYRUP/
JARABE EXPECTORANTE
118 ml / 4 fl oz