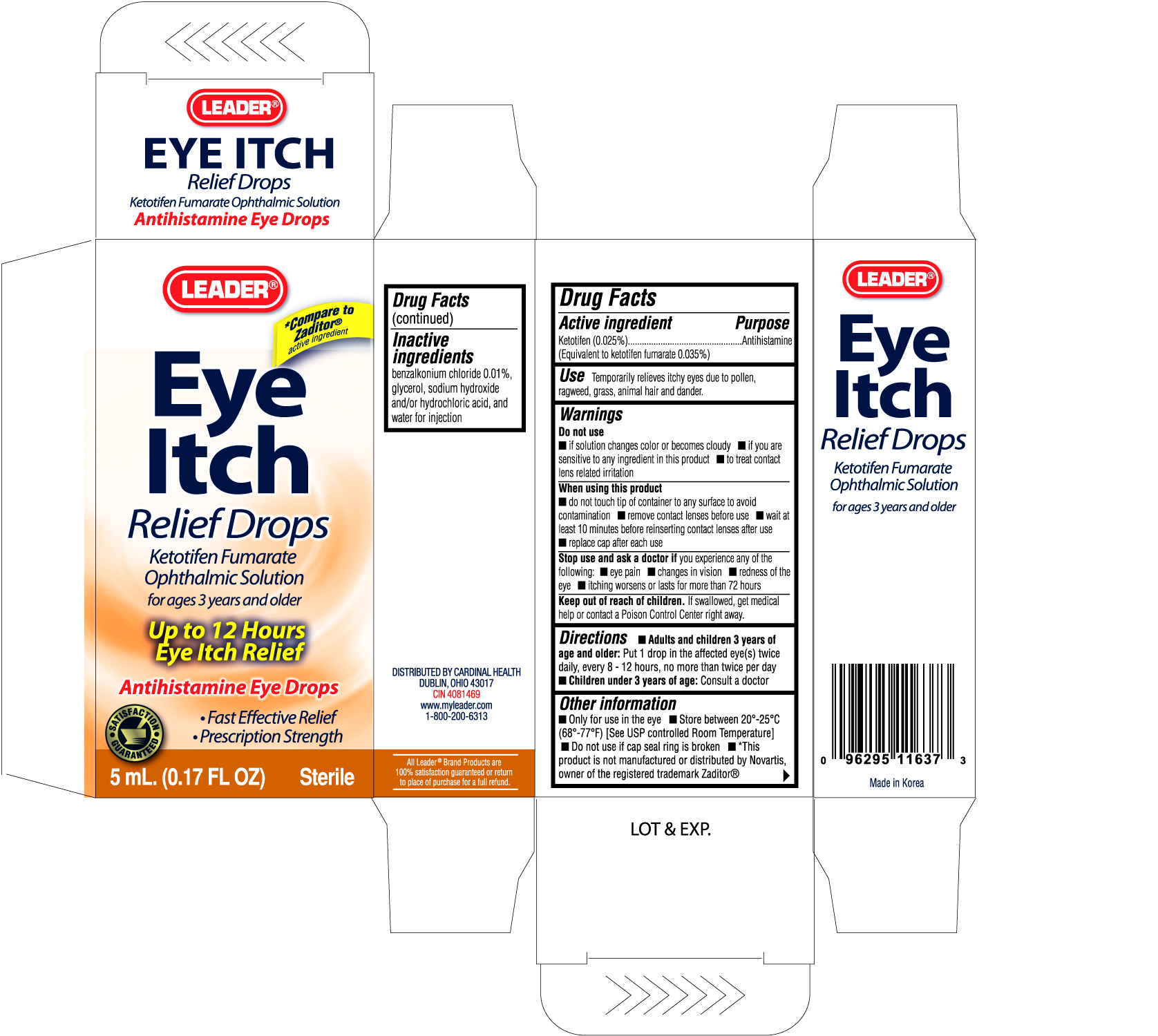
Active ingredient Purpose
Ketotifen (0.025%)..........................................Antihistamine
(Equivalent to ketotifen fumarate 0.035%)
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- redness of the eye
- itching worsens or lasts for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 3 years of age and older: Put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day
- Children under 3 years of age: Consult a doctor
Other information
- Only for use in the eye
- Store between 20o-25oC (68o to 77oF) (See USP controlled Room Temperature)
- Do not use if cap seal ring is broken
 Enter section text here
Enter section text here