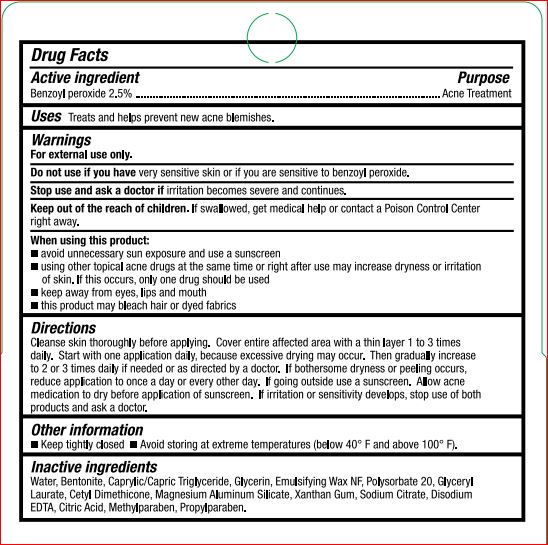
Keep out of reach of children.
If swallowed, get medical help or contact a Poison COntrol Center right away.
When using this product
- avoid unnecessary sun exposure and use a sunscreen
- using other topical acne drugs at the same time or right after use may increase dryness or irritation of skin. If this occurs, only one drug should be used.
- keep away from eyes, lips, mouth
- this product may bleach hair or dyed fabrics
Directions
Cleanse skin thoroughly before applying. COver entire affected area with a thin layer 1 to 3 times daily. Start with one application daily, because excessive drying may occur. Then gradually increase to 2 to 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. If going outsie use a sunscreen. Allow acne medication to dry before application of sunscreen. If irritation or sensitivity develops, stop use of both products and ask a doctor.
Other information
- Keep tightly closed
- Avoid storing at extreme temperatures (below 40 F and above 100 F).