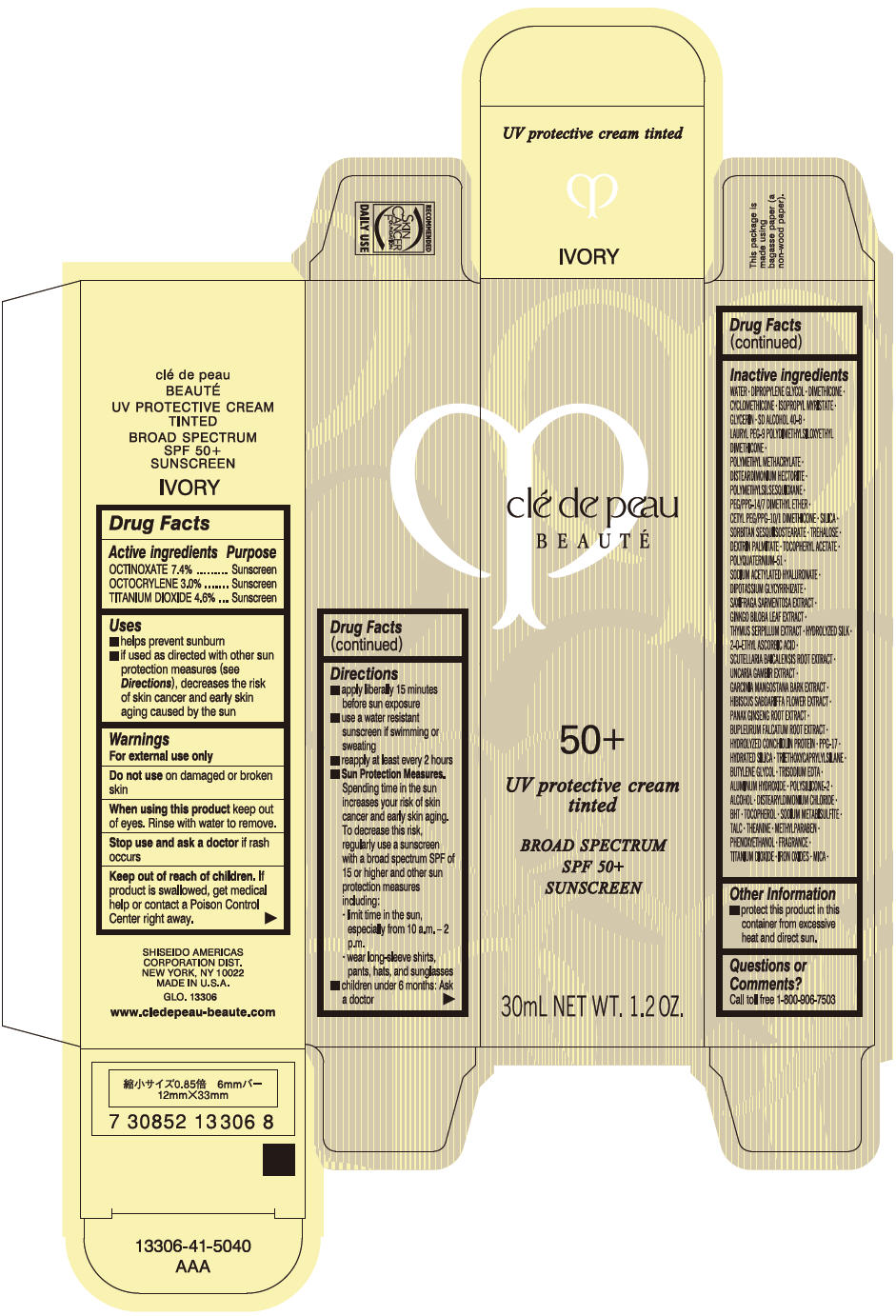
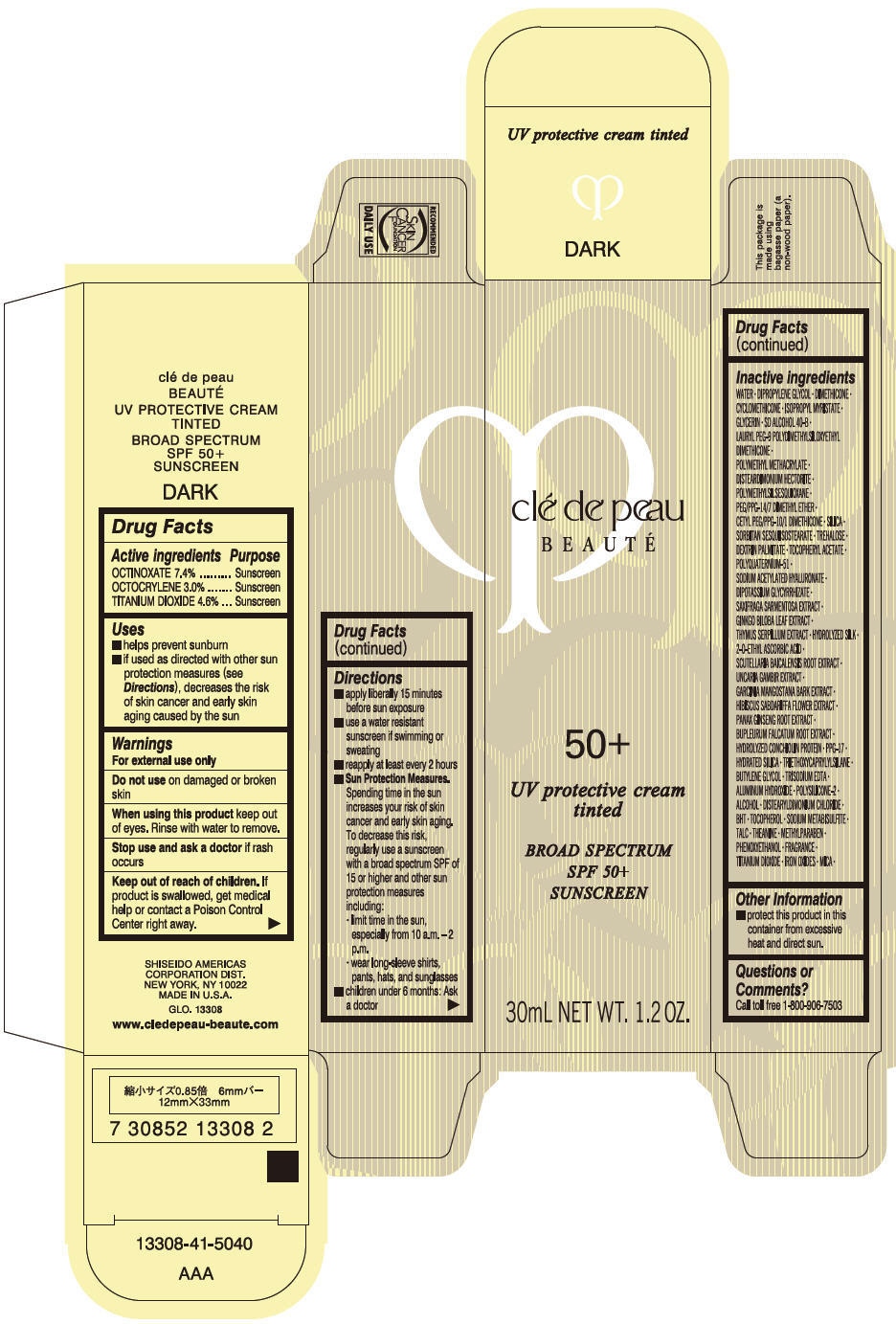
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER•DIPROPYLENE GLYCOL•DIMETHICONE•ISOPROPYL MYRISTATE•GLYCERIN•CYCLOPENTASILOXANE•SD ALCOHOL 40-B•LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE•METHYL METHACRYLATE CROSSPOLYMER•DISTEARDIMONIUM HECTORITE•POLYMETHYLSILSESQUIOXANE•PEG/PPG-14/7 DIMETHYL ETHER•CETYL PEG/PPG-10/1 DIMETHICONE•SILICA•SORBITAN SESQUIISOSTEARATE•TREHALOSE•DEXTRIN PALMITATE•TOCOPHERYL ACETATE•POLYQUATERNIUM-51•SODIUM ACETYLATED HYALURONATE•DIPOTASSIUM GLYCYRRHIZATE•SAXIFRAGA SARMENTOSA EXTRACT•GINKGO BILOBA LEAF EXTRACT•THYMUS SERPYLLUM EXTRACT•HYDROLYZED SILK•2-O-ETHYL ASCORBIC ACID•SCUTELLARIA BAICALENSIS ROOT EXTRACT•UNCARIA GAMBIR EXTRACT•GARCINIA MANGOSTANA BARK EXTRACT•HIBISCUS SABDARIFFA FLOWER EXTRACT•PANAX GINSENG ROOT EXTRACT•BUPLEURUM FALCATUM ROOT EXTRACT•HYDROLYZED CONCHIOLIN PROTEIN•PPG-17•HYDRATED SILICA•TRIETHOXYCAPRYLYLSILANE•BUTYLENE GLYCOL•TRISODIUM EDTA•ALUMINUM HYDROXIDE•POLYSILICONE-2•ALCOHOL•DISTEARYLDIMONIUM CHLORIDE•BHT•TOCOPHEROL•SODIUM METABISULFITE•TALC•THEANINE•METHYLPARABEN•PHENOXYETHANOL•FRAGRANCE•TITANIUM DIOXIDE•IRON OXIDES•MICA•
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - IVORY
clé de peau
BEAUTÉ
50+
UV protective cream
tinted
BROAD SPECTRUM
SPF 50+
SUNSCREEN
30mL NET WT. 1.2 OZ.